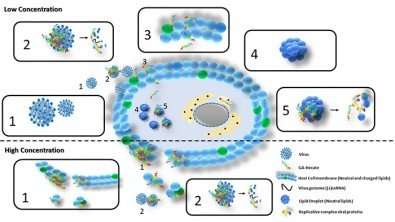
The compound called GA-Hecate also acts on bacteria, fungi and cancer cells and will be tested against Zika and yellow fever viruses (action mechanism proposed for GA-Hecate. Upper half of figure (low concentrations of material): (1) HCV; (2) interaction between GA-Hecate and viral envelope, destruction of virus and release of genetic material (+ssRNA); (3) interaction between GA-Hecate and host cell membrane, with permeabilization of peptide; (4) lipid droplet; (5) interaction between compound and lipid droplets, breaking down the latter and releasing replication complex proteins. Lower half (high concentrations of material): (1) interaction between compound and host cell plasma membrane; disruption resulting in cell death; (2) interaction with viral envelope and destruction of virus. Credit: Paulo Ricardo da Silva Sanches
A new compound that inhibits the replication of hepatitis C virus (HCV) in several stages of its lifecycle, and which is also capable of acting on bacteria, fungi and cancer cells, has been synthesized by researchers at São Paulo State University (UNESP) in Brazil.
The study was published in Scientific Reports, an online journal owned by Springer Nature.
"What we did was combine existing molecules by means of synthesis in the laboratory to produce new compounds with biological potential. This method is known as bioconjugation. Using bioconjugation, we synthesized six compounds and tested them on HCV genotypes 2a and 3a. The result was a compound with significant therapeutic potential," said Paulo Ricardo da Silva Sanches, a chemist and one of the two main authors of the study.
HCV displays considerable genomic variability, having at least six main genotypes, each of which has subtypes. Genotypes 2a and 3a are the most common HCV subtypes in circulation. The compound capable of destroying them is called GA-Hecate and was synthesized from gallic acid and the lytic peptide Hecate.
"We discovered that the compound acts on HCV in almost all stages of its reproductive cycle, which is unusual for antivirals. They generally have specific isolated targets, such as capsid proteins, membrane receptors or specific proteins such as NS3, inhibiting specific processes such as viral cell entry, the synthesis of genetic material and proteins, or the assembly and release of new viral particles. GA-Hecate, in contrast, showed broad activity encompassing several stages of the cycle," Sanches explained.
"The compound also displayed activity in lipid droplets, small lipid organelles in cells that are used by HCV during its replication and assembly and that protect the virus from attack by enzymes. GA-Hecate breaks these lipid droplets down and leaves the virus's replicative complex exposed to the action of cellular enzymes."
The researchers tested GA-Hecate both on the complete virus and on its "subgenome replicons," which contain all the elements required by the virus for replication of its genetic material in cells but are incapable of synthesizing the proteins responsible for infection. The compound was effective in all tests.
Another positive property of the compound is that it is highly selective, meaning it attacks the virus rather than the host cells. As such, it has the potential to be used as a drug for the treatment of the disease.
"Although the compound didn't display significant action on erythrocytes—red blood cells—the molecule has to undergo changes in its structure to reduce its toxicity still further," Sanches said. "This is what we're working on now, so that our research can move on from the in vitro stage to the in vivo stage."
According to Professor Eduardo Maffud Cilli, who supervised Sanches's Ph.D. research at UNESP's Chemistry Institute in Araraquara, "the average time it takes to plan and develop therapeutic peptides is 10 years. A study with this estimate has just come out. So far, approximately two years have been spent on developing the GA-Hecate molecule. Considering the statistical average, another eight years will be needed until the drug can come to market."
Cilli participated in the study and is also a co-author of the article published in Scientific Reports. "The great news is that this molecule doesn't act just on HCV. It can also act on bacteria, fungi and cancer cells," he said. "In addition, we're going to test the efficacy of GA-Hecate against Zika and yellow fever, since their replicative cycles are very similar to HCV's."
In the case of cancer, the molecule interacts with and destroys the membranes of affected cells. GA-Hecate's selectivity here is because cells modified by cancer have a larger quantity of surface negative charges than healthy cells, while the peptide is positively charged, so that its action is due to electrostatic attraction. In the case of the virus, the molecule's mechanism of action is more complex, as shown in the illustration.
More information: Mariana Nogueira Batista et al, GA-Hecate antiviral properties on HCV whole cycle represent a new antiviral class and open the door for the development of broad spectrum antivirals, Scientific Reports (2018). DOI: 10.1038/s41598-018-32176-w
Journal information: Scientific Reports
Provided by FAPESP