Kidney-resident macrophages—a role for healing during acute kidney injury?

During development in the womb, immune cells called macrophages go to the kidneys, and they remain there for life. Understanding the possible healing role for these macrophages after kidney damage may be crucial to helping treat patients who suffer acute kidney injury.
Acute kidney injury, or AKI, is a devastating condition that develops in two-thirds of critically ill patients, and patients with AKI have a 60 percent risk of dying. In AKI, kidneys can become scarred and can show progressive decline in function, becoming unable to heal their tissue.
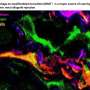
In a JCI Insight study published today, University of Alabama at Birmingham researchers have found that, during AKI in a mouse model, the kidney-resident macrophages are reprogrammed to a developmental state, resembling these same cells when they are found in newborn mice. Newborn mouse kidneys are still developing. This reprogramming during AKI may be important to promote healing and tissue regeneration. If a similar developmental shift is seen for human kidney-resident macrophages during AKI, that could aid new therapeutic approaches for patients.
The experimental challenge in this study was distinguishing the kidney-resident macrophages from the many cells that invade the kidney from blood circulation in response to kidney damage. Some of these invading cell types can differentiate into macrophages and dendritic cells in the kidney, and it was unknown whether some of the invaders become kidney-resident macrophages.
The UAB researchers used parabiosis—the linking of the blood circulatory systems between two mice—to reveal whether the kidney-resident macrophages after AKI were from invading precursors or from renewal by existing kidney-resident macrophages in the kidney.
In the parabiosis experiments, two mice shared blood circulation for four weeks, and then one underwent ischemia/reperfusion-induced AKI. Because the immune cells of the two mice have different surface markers that identify which mouse they come from, researchers could follow the invasion of the AKI kidneys by circulating immune cells from the healthy mouse. They found that the infiltrating cells contributed only minimally to the kidney-resident macrophage cell pool in the damaged kidneys.
Thus, after kidney injury, the kidney-resident macrophages are a distinct cellular subpopulation that does not differentiate from nonresident, infiltrating, precursor immune cells.
Researchers, led by co-senior authors Anupam Agarwal, M.D., director of the Division of Nephrology in the UAB Department of Medicine, and James George, Ph.D., professor in the UAB Department of Surgery, detailed how the kidney-resident macrophages are reprogrammed to a developmental state after injury. In response to the disease model, the kidney-resident macrophages turned off their expression of major histocompatibility complex type II, or MHCII. This lack of expression is similar to kidney-resident macrophages in newborn mice—those mice, the researchers showed, lack expression of this protein up to postnatal day seven, and then begin to express it over the next two weeks. Notably, MHCII protein and macrophages have important roles in autoimmunity and transplant rejection.
In addition, kidney-resident macrophages after AKI underwent transcriptional reprogramming to express a gene profile closely resembling that of the kidney-resident macrophages in newborn mice at postnatal day seven. Further supporting their role in development and healing, the reprogrammed kidney-resident macrophages were enriched in Wnt signaling, an active pathway that is vital for mouse and human kidney development.
Co-first authors of the JCI Insight study, "Resident macrophages reprogram toward a developmental state after acute kidney injury," are Jeremie M. Lever and Travis D. Hull, M.D., Ph.D., who are trainees from the NIH-funded UAB Medical Scientist Training Program, an M.D., Ph.D. program.
"Macrophage biology has reached a pivotal point," said Lever, a UAB graduate student and NIH F31 individual fellow. Many basic science research studies have suggested the importance for tissue-resident macrophages in healing after injury, but development of therapies promoting them is still in early stages, Lever says. "In order to successfully utilize these cells for contemporary translational interventions, I believe we will need to be specific about the origin—tissue-resident versus infiltrative—of the cells we plan to target."
Hull, now a surgery resident at Massachusetts General Hospital in Boston, said, "This work demonstrates that tissue resident macrophages possess the same plasticity that has been demonstrated in other immunological cell types. Moreover, this ability to reprogram to an early ontological phenotype is a potential avenue for therapeutic intervention, if the cellular signals and mechanisms of this reprogramming can be fully elucidated.
"This is an exciting development in the field of acute kidney injury," Hull said, "but also may represent a therapeutic target in fields such as transplantation, where the importance of macrophage biology is less well understood."
More information: Jeremie M. Lever et al, Resident macrophages reprogram toward a developmental state after acute kidney injury, JCI Insight (2019). DOI: 10.1172/jci.insight.125503