An unexpected mode of action for an antibody

Studies of human monoclonal antibodies isolated from survivors of coronavirus-induced severe acute respiratory syndrome (SARS) or Middle-East respiratory syndrome (MERS) are unveiling surprising immune defense tactics against fatal viruses. Atomic and molecular information about the workings of the highly potent antibodies may provide insights to prevent these serious and sometimes deadly lung infections.
Currently, no vaccines or specific treatments are available for any of the six coronaviruses that can infect humans. Some of these coronaviruses cause only common-cold like symptoms, but others provoke lethal pneumonias. Past deadly outbreaks in several countries foreshadow the possibility of coronavirus-mediated pandemics.
Additionally, genetic surveillance of coronaviruses in bats, and the fact that the MERS coronavirus naturally circulates in dromedary camels, suggest that previous outbreaks may not be unusual incidences. The animal/human species barrier is likely to be crossed again and lead to new emerging coronaviruses in the future.
As part of anticipation and preparation initiatives, infectious disease research groups are trying to develop an anti-coronavirus arsenal. An international team headed by UW Medicine scientists is among those attempting to understand how SARS and MERS coronaviruses infect humans, and how their presence elicits a response from the immune system. The research group is particularly interested in how neutralizing antibodies target the coronavirus' cell-invasion machinery.
Their most recent findings appear in the Jan. 31 online edition of Cell. The first authors are Alexandra Walls and Xiaoli Xiong, and the lead and senior author is David Veesler, all of the Department of Biochemistry at the University of Washington School of Medicine.
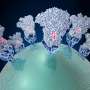
Coronaviruses have multi-functional surface spikes that recognize and attach to receptors on the surface of a host cell. They then fuse the virus and cellular membranes. They use these trimeric spike glycoproteins as their molecular break-in tool.
The spike glycoprotein densely decorates the surface of coronaviruses. The numerous projections resemble burrs on a seed-pod. The spikes are key to the infectivity and pathogenicity of the coronavirus. They are the target of neutralizing antibodies and the main focus of subunit vaccine design.
Previous studies in the Veesler lab at UW Medicine looked at the structural states that occur in the coronavirus spike before and after the membrane fusion reaction. The researchers saw large conformational changes in the spike glycoprotein. Details about activation of the membrane fusion cascade, however, remained unclear.
Using cryo-electron microscopy and other powerful technologies, the researchers gained insight into how the neutralizing monoclonal antibodies from the SARS and MERS survivors inhibit the viruses at the molecular level. Their findings also helped elucidate the unusual nature of coronavirus membrane fusion activation.
The researchers found that both the SARS and the MERS coronavirus antibodies blocked the virus spikes from interacting with the receptors on the host cell membrane.
The SARS coronavirus antibody also did something unexpected: it functionally mimicked receptor-attachment and induced the spike to undergo conformational changes leading to membrane fusion. This trigger seems to be driven by a molecular ratcheting mechanism.
"The finding is an unprecedented example of functional mimicry," the researchers noted, "whereby an antibody activates membrane fusion by recapitulating the action of the receptor."
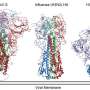
This study used molecular imaging to characterize the structures of both SARS and MERS coronavirus spike glycoproteins in a complex with their respective antibodies.
The researchers also provided a blueprint of the carbohydrates that bedeck the spike glycoproteins, in the context of whole viruses. Coronaviruses use this strategy to mask the vulnerable portion of their fusion apparatus to limit antibody access to it and expose it only to carry out the recognition and infection of host cells.
More information: Cell (2019). DOI: 10.1016/j.cell.2018.12.028
Journal information: Cell
Provided by University of Washington