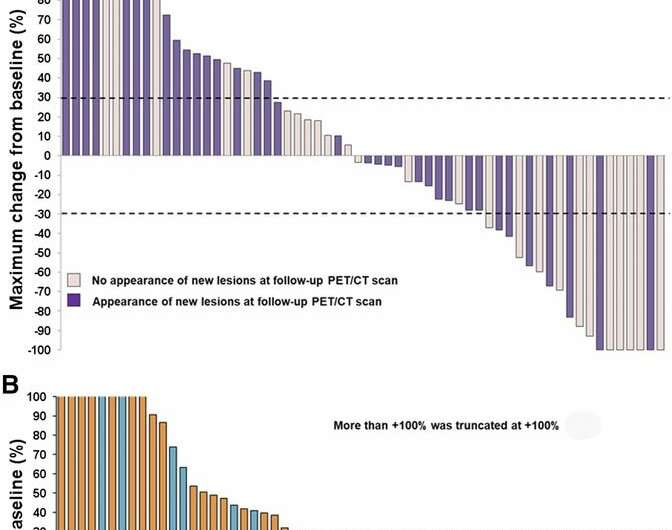
Waterfall plot of maximum changes in SULpeak for (A) PERCIST5, (B) PERCIST1, and (C) imPERCIST5. Upper short, dashed line indicates separation of progressive metabolic disease from stable metabolic disease. Lower short, dashed line indicates separation of stable metabolic disease from peak metabolic rate. Credit: Ito et al., Memorial Sloan Kettering Cancer Center, New York, New York.
Nuclear medicine imaging with PET/CT can monitor the effectiveness of immunotherapy treatment for metastatic melanoma and predict outcome. In this way, a patient's therapy can be more effectively tailored to his or her personal response.
Metastatic melanoma is one of the deadliest skin cancers, so determination of a treatment's effectiveness is essential but can be tricky. A study featured in the March issue of The Journal of Nuclear Medicine demonstrates that 18F-FDG positron emission tomography/computed tomography (PET/CT) can monitor immunotherapy with ipilimumab, a "checkpoint inhibitor" that allows the immune system to attack cancer cells.
"Checkpoint inhibitor therapy is now a standard therapy for metastatic melanoma," explains Wolfgang A. Weber, MD, of Technical University Munich, Germany (formerly of Memorial Sloan Kettering Cancer Center). "However, there were concerns about whether FDG PET/CT could be used to monitor tumor response to this immunotherapy, because inflammatory reactions to the immunotherapy may cause false positive findings. The present study shows that tumor response to checkpoint inhibitor therapy with ipilimumab can be assessed accurately by FDG PET after completion of ipilimumab therapy."
Clinical studies have shown that ipilimumab, when compared with chemotherapy, can significantly improve the survival of patients with metastatic melanoma; however, this is true for only 15 to 20 percent of melanoma patients. The ability to accurately assess a patient's response to ipilimumab therapy would allow physicians to adjust his or her course of treatment for maximum effectiveness.
To overcome the difficulties of assessing tumor response to ipilimumab and other checkpoint inhibitors, the researchers developed new response criteria. These new criteria require confirmation of tumor progression on a follow-up scan.
For this retrospective study, 60 patients with metastatic melanoma received FDG PET/CT scans pre- and post-treatment. Tumor response was assessed by the change in the sum of SULpeak (standard uptake value normalized to lean body mass) of up to 5 lesions (PERCIST5). A second analysis (PERCIST1) was done of the lesion with the highest SULpeak between the baseline and follow-up scan. New lesions on PET that appeared suspicious for metastases were considered progressive metabolic disease. To assess new inflammatory lesions, the team applied their novel immunotherapy-modified PERCIST with a 5-lesion analysis (imPERCIST5). In imPERCIST5, a new lesion on FDG PET/CT is considered progressive disease only if it increases the sum of SULpeak by more than 20%.
The results of the study show that assessment of tumor response to ipilimumab treatment using PERCIST correlated significantly with survival of patients with advanced melanoma. The slight modifications of PERCIST to imPERCIST5 further improved the prognostic value of response assessment by 18F-FDG PET/CT.
Weber points out, "FDG PET/CT is routinely used to stage melanoma. The present study suggests that it also can be used to monitor tumor response to ipilimumab therapy and predict outcome. FDG PET can identify patients with favorable and unfavorable prognoses—leading to therapy escalation (e.g., combination immunotherapy) or de-escalation (e.g., reduced number of therapy cycles)." In this way, a patient's therapy can be more effectively tailored to his or her personal response.
More information: Kimiteru Ito et al, 18F-FDG PET/CT for Monitoring of Ipilimumab Therapy in Patients with Metastatic Melanoma, Journal of Nuclear Medicine (2018). DOI: 10.2967/jnumed.118.213652
Journal information: Journal of Nuclear Medicine
Provided by Society of Nuclear Medicine and Molecular Imaging