The arrestin-GPCR connection
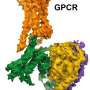
G-protein coupled receptors (GPCRs) are the "inbox" of environmental messages in mammalian cells. Because of their central role in signaling pathways, mutations resulting in abnormal GPCR functions cause a wide variety of diseases. Therefore, GPCRs are the most intensively studied drug targets.
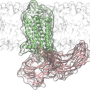
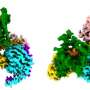
After activation, GPCRs need to be deactivated by enzymes called GPCR kinases and a family of proteins called arrestins. Intriguingly, while there are hundreds of GPCR subtypes, mammals have only four arrestins. One region where arrestin subtypes differ is the protein's "finger loop" in the center of the receptor-binding surface.
Now in a study published in the journal PLOS ONE, Vsevolod Gurevich, Ph.D., and colleagues show that a glycine residue at the beginning of the finger loop is critical for the arrestin-GPCR interaction.
Their work demonstrates that the ability of arrestin to "mold" itself to fit into the GPCR complex is more important for receptor binding than the actual protein sequence in other arrestin elements.
More information: Chen Zheng et al. Critical role of the finger loop in arrestin binding to the receptors, PLOS ONE (2019). DOI: 10.1371/journal.pone.0213792
Journal information: PLoS ONE
Provided by Vanderbilt University