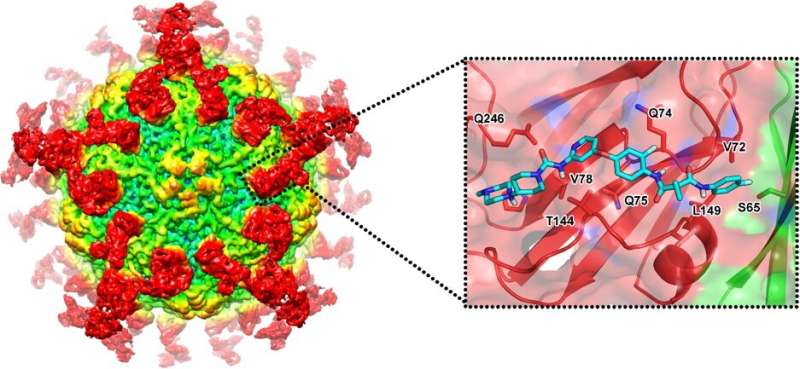
Rational drug design based on structural information of epitopes on HAV capsids. Credit: Xiangxi Wang
Structure-based drug design revealed that a compound previously investigated for the treatment of head-and-neck cancer could function as a lead compound for the development of drugs to treat hepatitis A virus infection, according to a study publishing April 30 in the open-access journal PLOS Biology by Dan Su of Sichuan University, Zihe Rao of Tsinghua University, Xiangxi Wang of the Chinese Academy of Sciences, and colleagues.
Hepatitis A caused by is a picornavirus that infects approximately 1.5 million people annually and continues to cause substantial mortality. Although there are effective vaccines, antivirals against hepatitis A infection are still required during outbreaks, and to date there are no licensed therapeutic drugs. The authors hypothesized that better knowledge of how neutralizing antibodies naturally defend cells from hepatitis A infection might facilitate the development of hepatitis A-targeting antiviral drugs.
In the new study, the authors report four potent hepatitis A virus-specific neutralizing antibodies, together with their previously reported one, that efficiently inhibit hepatitis A virus infection by blocking the virus's ability to attach to the host cell. The authors used high-resolution cryogenic electron microscopy to visualize structures of hepatitis A virus bound to the antibodies. This gave them insights into the structural basis of how the antibodies neutralize the virus, informing the rational design of highly potent inhibitors.
Using molecular modeling, the authors then identified one promising inhibitor, named golvatinib, from the DrugBank Database. In vitro assays confirmed its ability to block viral infection and unveiled its neutralizing mechanism. According to the authors, this combined experimental and computational approach could be useful in the rational design of effective drugs for picornavirus infections.
More information: Cao L, Liu P, Yang P, Gao Q, Li H, Sun Y, et al. (2019) Structural basis for neutralization of hepatitis A virus informs a rational design of highly potent inhibitors. PLoS Biol 17(4): e3000229. doi.org/10.1371/journal.pbio.3000229
Journal information: PLoS Biology
Provided by Public Library of Science