Researchers continue efforts to lower drug-resistant hypertension without medication
The Emory Heart & Vascular Center is participating in an FDA-approved pivotal trial, called CALM-2 (Controlling And Lowering blood pressure with MobiusHDÒ), of a novel catheter-based, non-surgical procedure to treat patients with drug-resistant hypertension.
Many people struggle to control their blood pressure to targeted levels, despite taking multiple antihypertensive drugs. The need for a non-pharmaceutical treatment has become critically important, and Emory is active in the clinical research of promising new treatments.
"Uncontrolled high blood pressure is a tremendous unmet clinical need and is the most important single risk factor for cardiovascular morbidity and death," says cardiologist Chandan Devireddy, MD, associate professor of medicine at Emory University School of Medicine. "A safe treatment that can lower dangerously high blood pressure levels for those who have failed drug therapy could present a life-changing option for them."
The pivotal CALM-2 trial is a continuation of an earlier proof-of-concept CALM-FIM study of the passive MobiusHD implant in patients with uncontrolled high blood pressure. Led by Devireddy in 2013, Emory was the first investigative site in the world to implant the device. The results of the CALM-FIM study, reported in The Lancet in 2017, showed significant reductions in blood pressure through six months. These encouraging results led to the design of the larger, multi-center CALM-2 clinical trial intended to generate definitive evidence of safety and effectiveness of this novel procedure.
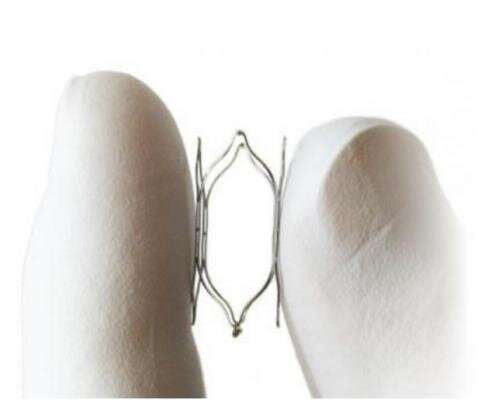
Specialized stretch-sensitive nerves called baroreceptors are located in the walls of the carotid arteries and play an essential role in the body's natural blood pressure regulation. The MobiusHD system is the first minimally invasive procedure to utilize the baroreceptor mechanism to address uncontrolled hypertension.
"The initial results using the MobiusHD implant to reshape the carotid sinus and stretch baroreceptors to trigger the body's natural blood pressure regulation are encouraging and continue to be of great interest to us," adds Devireddy. "The safety and efficacy for this procedure need to be confirmed, and we look forward to being an integral part of the pivotal CALM-2 trial."
The CALM-2 clinical trial, sponsored by Vascular Dynamics Inc., is currently enrolling and is seeking up to 300 drug-resistant hypertension patients at select medical centers around the U.S. and in the UK and Europe, including Emory.
More information: For more information on the CALM-2 trial at Emory, please call 404-686-7478. To learn more about the CALM-2 study, visit:
clinicaltrials.gov.