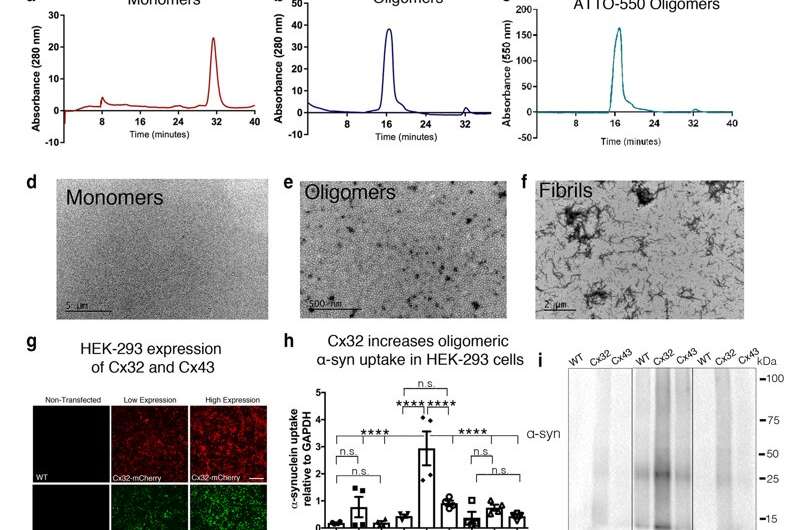
Fig. 1 Cx32 facilitates the uptake of oα-syn preferentially to monomers or fibrillar assemblies. a SEC analysis of α-syn monomers or b oligomeric α-syn (oα-syn) assemblies at 280 nm absorbance. c SEC confirmation of labeled ATTO-550 oα-syn assemblies at 550 nm absorbance. d TEM characterization of α-syn monomers, e oligomers, or f fibrillar assemblies; scale bars represent 5 µm, 500 nm, and 2 µm, respectively. g Confocal image analysis of non-transfected wild-type (WT) or transfected HEK-293 cells with low (15 µg) or high (50 µg) expression of Cx32-mCherry or Cx43-GFP plasmid constructs. Scale bars represent 200 µm. h Densitometric analysis of i Western blot of monomeric, oligomeric or fibrillar α-syn uptake in WT HEK-293 cells or HEK-293 cells expressing Cx32 or Cx43 (n = 4, two-way ANOVA followed by Tukey’s post hoc test for multiple comparisons, n.s. = no significance, F(8, 24) = 13.1, ****p < 0.0001). j Densitometric analysis of k representative Western blots of oα-syn uptake in differentiated SH-SY5Y WT cells or SH-SY5Y cells expressing Cx26, Cx32, Cx43 or Cx32-KO, (n = 5, one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons, n.s; no significance, F(4, 24) = 25.28, ****p < 0.0001). l Immunocytochemistry of differentiated SH-SY5Y cells, WT and those expressing Cx26, Cx32, Cx43 or Cx32-KO treated with oα-syn assemblies. Scale bars represent 20 µm. m Densitometric analysis of n representative Western blot of fibrillar α-syn uptake in differentiated SH-SY5Y cells, WT and those expressing Cx26, Cx32, Cx43, or Cx32-KO (n = 3, one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons, n.s; no significance, F(4, 12) = 76.16, *p < 0.05, ****p < 0.0001)
Tiny channels between nerve cells are involved in a newly discovered mechanism of how Parkinson's disease can spread throughout the brain, according to new research from Linköping University, Sweden. The results demonstrate that harmful protein aggregates, or deposits, can bind and "hitch a lift" with channel-forming proteins, and in this way spread to healthy cells. The study has been published in Acta Neuropathologica.
Neurodegenerative diseases, such as Alzheimer's, Parkinson's and Huntington's disease, affect different regions of the human brain. Despite these regional differences, research has shown that the processes inside cells affected by these diseases have a lot in common. One characteristic of these diseases is that specific proteins start to form aggregates that damage and eventually kill the cell. In Parkinson's disease, it is misfolded forms of a protein known as α-synuclein that are involved. These aggregates can recruit normal forms of α-synuclein, causing the formation of more protein aggregates.
"During the past few decades, we have realised that the protein deposits in the brain can spread between cells, acting as seeds that start a new aggregation cycle in the next cell. The disease in this way spreads in the brain in a manner similar to an infection. We want to understand how the protein spreads, and use this knowledge in the long term to inhibit the spread of disease in the brain," says Martin Hallbeck, associate professor in the Department of Clinical and Experimental Medicine at Linköping University and consultant in surgical pathology at Linköping University Hospital.
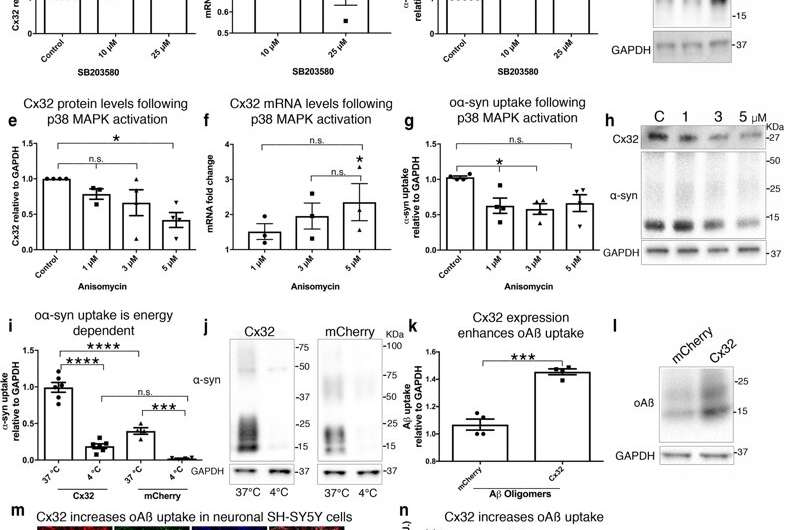
Fig. 2 Cx32 expression correlates with oα-syn uptake. a Densitometric analysis of Cx32 protein levels in differentiated SH-SY5Y cells exposed to SB203580 for 24 h (10 and 25 µM), (n = 5, one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons, n.s; no significance, F(2, 12) = 5.547, *p < 0.05). b qRT-PCR analysis of Cx32 mRNA in differentiated SH-SY5Y cells with increasing SB203580 concentrations and untreated cells as controls (n = 3, one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons, n.s. = no significance F(3, 8) = 1.415). c Densitometric analysis of oα-syn uptake in differentiated cells exposed to SB203580 for 24 h (n = 5, one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons, n.s; no significance, F(2, 12) = 4.562, *p < 0.05). d Representative Western blots showing Cx32, α-syn and GAPDH protein levels following SB203580 treatment. e Densitometric analysis of Cx32 protein expression in differentiated SH-SY5Y cells exposed to anisomycin for 24 h, (n = 5, one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons, n.s; no significance, F(3, 11) = 4.553, *p < 0.05). f qRT-PCR analysis of Cx32 mRNA in differentiated SH-SY5Y cells treated with increasing anisomycin concentrations and untreated cells as controls (n = 3, one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons, n.s; no significance, F(3, 8) = 3.998, *p < 0.05). g Densitometric analysis of oα-syn uptake following anisomycin treatment for 24 h (n = 4, one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons, n.s; no significance, F(3, 12) = 5.327, *p < 0.05). h Representative Western blots showing Cx32, α-syn and GAPDH protein levels following anisomycin treatment. i, j Western blot analysis of oα-syn uptake in SH-SY5Y cells expressing Cx32-mCherry (n = 6) or mCherry (n = 4) incubated at 37 °C or 4 °C (one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons, n.s; no significance, F(3, 16) = 83.02, ***p < 0.001, ****p < 0.0001). k Densitometric analysis of l Western blots of oAβ uptake in differentiated SH-SY5Y cells expressing mCherry or Cx32-mCherry (n = 4, unpaired, two-tailed t test, t6 = 8.493, ***p = 0.0001). m Confocal image analysis of differentiated SH-SY5Y cells expressing mCherry or Cx32-mCherry (red) labeled with Aβ (6E10, green) and then stained with Hoechst (blue); scale bar represents 200 µm. n Fluorescence intensity quantification of oAβ uptake in differentiated SH-SY5Y cells expressing mCherry or Cx32-mCherry by confocal microscopy (n = 3 independent experiments and each representing the average of three different measurements, unpaired, two-tailed t test, t4 = 11.86 ***p < 0.001)
It has long been known that cells that lie close to each other can create small channels (known as gap junction channels) between them. These small channels are built from members of a family of proteins known as connexins. Studies by other scientists have suggested that connexins play a role in other types of disease, such as HIV/AIDS. This led the researchers at Linköping University to wonder whether connexins can play a similar role in the spread of Parkinson's disease in the brain.
"It turned out that the harmful protein aggregates in Parkinson's disease can bind to connexin-32, Cx32, and be absorbed by a cell. We are the first to demonstrate that connexins play a role in the uptake and transfer of disease-related proteins from one cell to another in Parkinson's disease and multiple system atrophy, a related disorder," says Juan Reyes, senior postdoc in Martin Hallbeck's research group and primary author of the article.
The brain contains more than 10 connexins, but the study suggests that the protein deposits in Parkinson's disease interact with only one of them, Cx32. Details of the process by which the harmful proteins transfer from one cell to a neighbouring cell with the aid of the channel-forming protein remain unclear. The scientists do know that the channel created by connexin is too narrow for the protein aggregates to pass through. They have shown that the aggregates bind to the channel-forming protein Cx32 and sneak into the cell together with it. When the researchers inhibited the formation of channels in cells in culture, absorption of α-synuclein was prevented. In experiments using brain tissue from four deceased patients diagnosed with Parkinson's disease, the scientists observed a direct binding between synuclein and connexin in two of the cases, which suggests that they interact with each other also in the Parkinsonian brain but not in normal brains.
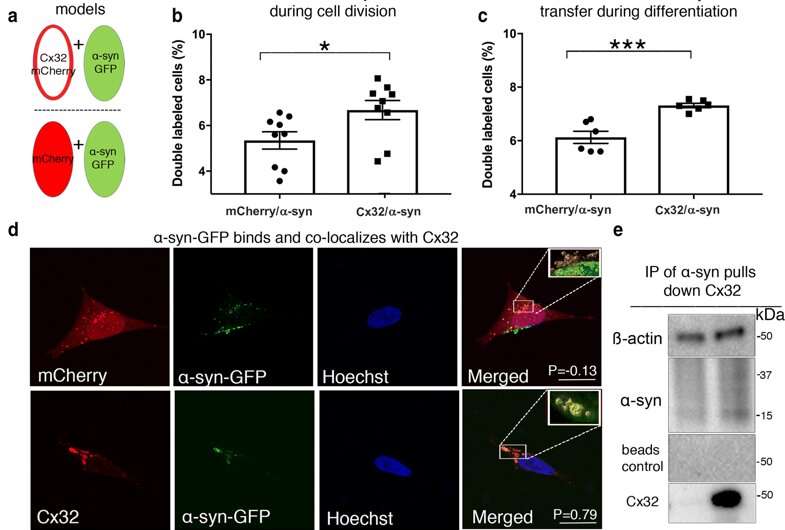
Fig. 3 Cx32 interacts with oα-syn and facilitates uptake and transfer in neurons and oligodendrocytes. a Schematic representation of the co-culture donor and recipient SH-SY5Y models designed to quantify α-syn-GFP transfer as donor cells (green) and recipient cells shown in red by open (Cx32-mCherry) or closed circle (mCherry), depicting the relative expression of the plasmids. b Flow cytometry measurement of α-syn-GFP transfer in undifferentiated conditions (n = 9 independent experiments and each representing the average of triplicate measurements, unpaired, two-tailed t test, t16 = 2.35, *p < 0.031). c Flow cytometry analysis of α-syn-GFP transfer in a differentiated state (n = 6 independent experiments and each representing the average of duplicate measurements, unpaired, two-tailed t test, t10 = 4.03, ***p < 0.001). d Confocal image analysis of recipient cells (mCherry and Cx32-mCherry) containing α-syn-GFP oligomers sorted by FACS and analyzed by Huygens Pro (top 3D insert), demonstrating colocalization between Cx32 and α-syn-GFP as indicated by the Pearson correlation coefficient (P) of 0.79 compared to -0.13 for α-syn-GFP and mCherry; scale bars represent 20 µm. e Immunoprecipitation (IP) of oα-syn followed by Western blot analysis with mCherry and α-syn antibodies identifies an interaction between oα -syn and Cx32 f Densitometric analysis of g representative Western blots of α-syn uptake in monomeric, oligomeric or fibrillar assemblies in differentiated WT rat oligodendrocytes (OLN-93) or oligodendrocytes expressing Cx32-mCherry or cells lacking Cx32 (Cx32-KO) (n = 6, two-way ANOVA followed by Tukey’s post hoc test for multiple comparisons, n.s; no significance, F(8, 24) = 6.13, *p < 0.05, **p < 0.01 ***p < 0.001). h Immunocytochemistry of OLN-93 cells overexpressing Cx32-mCherry showing the typical Cx32-mCherry plaques at the cell membrane (red, arrowheads), or cell soma (*) co-labeled with Cx32 antibody (green) and Hoechst (blue), scale bars represent 10 µm. Top insert represents a 3D image demonstrating the Cx32 plaque at the cellular membrane (arrow) and cytoplasm (*) i Immunocytochemistry and orthogonal view of human oligodendrocyte precursor cells (OPCs) overexpressing Cx32-mCherry (at the cell membrane, arrowheads) stained with Hoechst (blue) and CNPase (gray); scale bars represent 10 µm. j Densitometric analysis of k representative Western blot of α-syn uptake (monomeric, oligomeric or fibrillar) in differentiated human WT oligodendrocytes or oligodendrocytes overexpressing Cx32-mCherry (n = 4, two-way ANOVA followed by Tukey’s post hoc test for multiple comparisons, n.s; no significance, F(5,15) = 5.914, *p < 0.05, **p < 0.01)
"We hope that connexin-32 can be used in the future as a target for drug treatment," says Martin Hallbeck.
Parkinson's disease is the second most common neurodegenerative disease, after Alzheimer's disease. The condition is characterised by tremors, stiff muscles and difficulties is starting physical activity, which becomes slow. Cognitive symptoms are also common in later stages of the disease. When symptoms become apparent, a relatively large fraction of the nerve cells in the region of the brain affected have already died. No treatment is currently available to inhibit disease progression.
More information: Juan F. Reyes et al. Binding of α-synuclein oligomers to Cx32 facilitates protein uptake and transfer in neurons and oligodendrocytes, Acta Neuropathologica (2019). DOI: 10.1007/s00401-019-02007-x
Provided by Linköping University