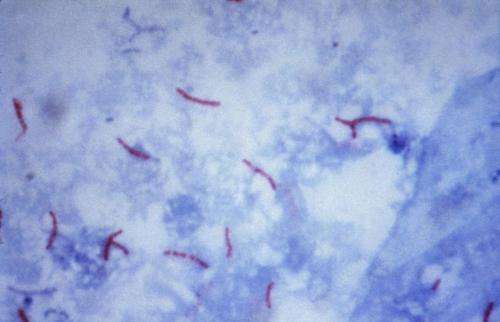
This photomicrograph reveals Mycobacterium tuberculosis bacteria using acid-fast Ziehl-Neelsen stain; Magnified 1000 X. The acid-fast stains depend on the ability of mycobacteria to retain dye when treated with mineral acid or an acid-alcohol solution such as the Ziehl-Neelsen, or the Kinyoun stains that are carbolfuchsin methods specific for M. tuberculosis. Credit: public domain
Researchers have made a breakthrough that could eventually lead to a more effective treatment for tuberculosis.
Tuberculosis is one of the top 10 causes of death worldwide, according to the World Health Organization.
A team of scientists, including Professor Colin Jackson from The Australian National University (ANU), has solved the mystery of how a cofactor called F420, found in the bacterium behind tuberculosis, is made.
Cofactors like F420 help enzymes to speed up chemical reactions.
Professor Jackson said this breakthrough could help identify new drug targets for tuberculosis.
"F420 is found in Mycobacterium tuberculosis—the bacteria which causes tuberculosis. But is not synthesised by the human body," Professor Jackson said.
"For decades, people have been unsure about how F420, has been made. We were able to go through and identify all the different enzymes involved in making this cofactor.
"Understanding its make-up could allow scientists to better target the disease in patients. This is particularly significant as TB is the world's deadliest infectious diseases, claiming over one million lives each year."
Now researchers know how the cofactor is made in Mycobacterium tuberculosis, they can also produce it in other organisms—helping unlock safer and cleaner biotechnology applications.
"This is really important for what we call 'green chemistry'," Professor Jackson said.
"Instead of manufacturing a certain chemical using toxic solvents or high heat, we can now use enzymes that use this cofactor to do it in more environmentally-friendly conditions."
Their research has been published in the journal Nature Communications.
More information: Ghader Bashiri et al. A revised biosynthetic pathway for the cofactor F420 in prokaryotes, Nature Communications (2019). DOI: 10.1038/s41467-019-09534-x
Journal information: Nature Communications
Provided by Australian National University