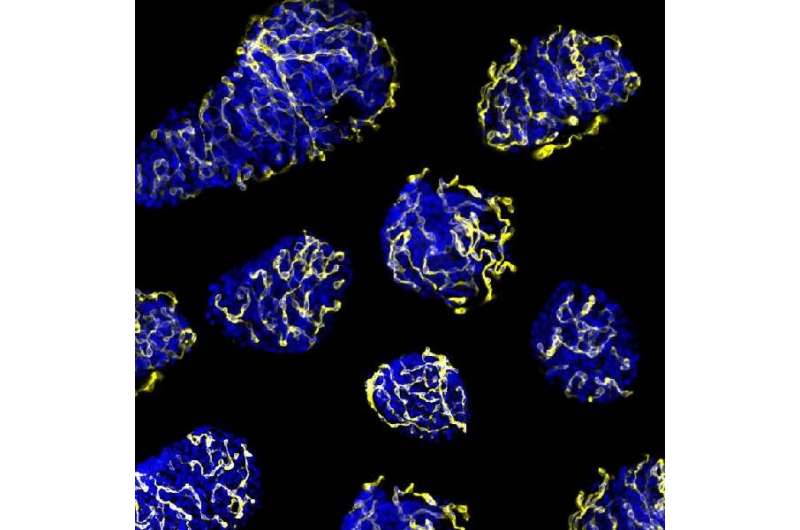
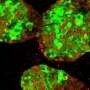
The inhibition of the PTP1B phosphatase activity makes the transplanted beta cells in the pancreas survive and fulfill their function, inducing regular sugar levels. Credit: Figueiredo et al.
The primary cause of the loss of functionality in transplanted pancreatic islets is their low capacity to create new vessels to transport nutrients. Researchers from the University of Barcelona and IDIBAPS have led a study that identifies a protein as the potential modulator in the revascularization of pancreatic islets.
In the study, conducted on diabetic mice with islet transplant from other animals or human islets, researchers showed that grafts without this protein have a higher revascularization, favouring the viability of cells. Regular sugar levels and glucose tolerance were also recovered.
The study was coordinated by Ramon Gomis, professor at the Faculty of Medicine and Health Sciences of the University of Barcelona, and Rosa Gasa, researcher in the same group. The first author of the study, published in the journal Science Translational Medicine, is Hugo Figueiredo, researcher in the IDIBAPS group.
Regenerative medicine for type 1 diabetes treatment
One of the used strategies in type 1 diabetes treatment based on regenerative medicine is pancreatic islet transplantation. Islets are formed by different types of cells and produce hormones such as insulin and glucagon. In type 1 diabetes, beta cells in islets responsible for the production of insulin are selectively destroyed by an autoimmune process.
For this reason, islet transplantation can restore physiological function in patients with this type of diabetes. "Although this transplant is carried out in some centers, it has limitations, such as the chronic administration of immunosuppressants, and it is applied in those cases in which the disease is not properly controlled. Currently, it is indicated in the context of a kidney transplant, and we opt for a dual vascular kidney-pancreas transplant," says Ramon Gomis, coordinator of the study.
There are two basic challenges involved:the chronic administration of immunosupressants and the fact that the transplant is not re-vascularized properly, which makes it harder for transport of nutrients and oxygen. This deficiency makes the islets nonviable and causes them to die.
The regular environment for islets is formed by a heavy network of capillaries in charge of the arrival of oxygen, hormones and nutrients and the transport of the generated hormones to the blood vessel. During the transplant process, islets are separated from their vascular network and their right function and survival depends on the ability to create new vessels for the vascular system in the receptor.
"Islet implantation is revascularized, but not fast enough. In the article, we focused on getting enough vessels to keep the islets in optimal condition and improve the success of this strategy for the treatment of type 1 diabetes," notes researcher Rosa Gasa, coordinator of the study.
Improving the revascularization
In the study, the researchers identified a molecular target –PTP1B phosphatase- which would make the transplanted pancreatic islets viable. The results show the inhibition of this enzyme, which is present in all cells including pancreatic beta cells, promotes the activity of the pro-angiogenic growth factor VEGF, which enables the creation of new blood vessels. Therefore, it gives a higher re-vascularization of the treatment that improves islet survival and functionality.
"Regulation of the re-vascularization is induced by hypoxia and the lack of nutrients, and the inhibition of phosphatase widens this response. When the stimuli disappears, the creation of new blood vessels stops," notes Rosa Gasa.
"This article is a proof of concept that removes one of the reasons that pancreatic islet transplant fails. There are less specific inhibitors of PTP1B and phosphatases, and therefore, the next step will be to test these inhibitors in the islet transplant in humans and assess its success," concludes Ramon Gomis.
More information: Hugo Figueiredo et al. Targeting pancreatic islet PTP1B improves islet graft revascularization and transplant outcomes, Science Translational Medicine (2019). DOI: 10.1126/scitranslmed.aar6294
Journal information: Science Translational Medicine
Provided by University of Barcelona