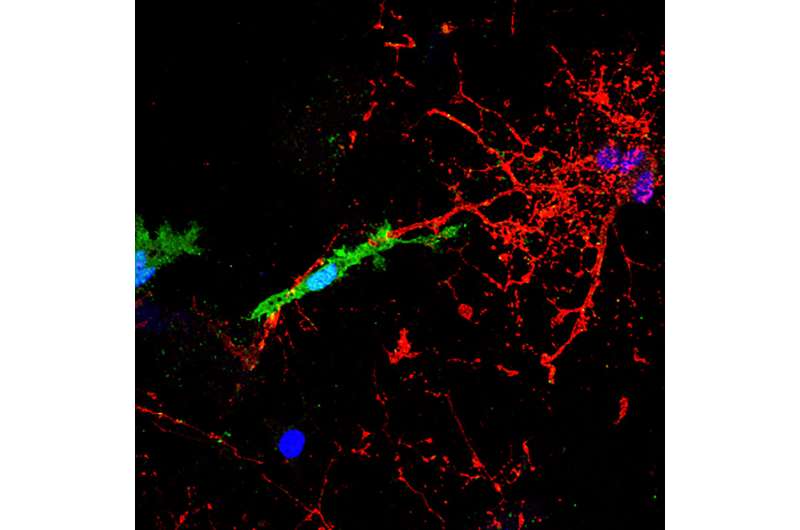
A microglial cell, labelled in green, contacts and attacks a myelinated axon (in red). In the presence of the pHERV-W envelope protein, this interaction leads to axonal injury. The blue structures are cell nuclei. Credit: HHU / Joel Gruchot / Patrick Küry
Early disease stages of multiple sclerosis (MS) are primarily characterized by immune cell infiltration of the central nervous system (CNS). This causes inflammation that damages the myelin sheaths that insulate nerves, which are established by specialized glial cells of the CNS called oligodendrocytes. These structures protect, nourish and stabilize the axons that transmit electrical signals between neurons.
There is a large therapeutic repertoire of immunomodulatory drugs available that can effectively target the inflammatory aspects of relapsing multiple sclerosis (RMS). But when MS progresses, damage accumulates, which ultimately results in irreversible deficits and clinical disability. Unfortunately, despite decades of intense research, disease progression is still untreatable, as there are no therapies available that either prevent damage or repair injured axons.
In a new study published on June 18 in PNAS, a research team led by Professor Dr. Patrick Küry from the Department of Neurology (chaired by Professor Dr. Hans-Peter Hartung) has shed light on a novel axon damage mechanism that could be highly relevant for progressive MS (PMS) patients.
As outlined by first author Dr. David Kremer, the envelope (ENV) protein of the pathogenic human endogenous retrovirus type W (pHERV-W) was found to be a major contributor to nerve damage in MS. In collaboration with research teams in the U.S. and Canada, the authors demonstrated that the ENV protein drives CNS resident microglial cells to contact and damage myelinated axons.
Alongside the scientific research into the damage mechanism, clinical developments aiming at neutralizing the harmful ENV protein in MS patients have also progressed. Two clinical studies conducted under the supervision of Professor Hartung have already successfully tested the ENV-neutralizing antibody temelimab. MRI scans of the participants treated in the study showed reduced damage to the nerve tissue.
The Düsseldorf-based researchers and their colleagues can therefore explain why neurodegeneration is decreased in patients treated with temelimab. This antibody specifically binds to the ENV protein of the retrovirus and blocks its activity in the CNS. Professor Hartung says future clinical studies in progressive MS patients will now have to demonstrate whether temelimab treatment can also improve clinical symptoms resulting from neurodegeneration.
More information: David Kremer et al, pHERV-W envelope protein fuels microglial cell-dependent damage of myelinated axons in multiple sclerosis, Proceedings of the National Academy of Sciences (2019). DOI: 10.1073/pnas.1901283116
Journal information: Proceedings of the National Academy of Sciences
Provided by Heinrich-Heine University Duesseldorf