Credit: CC0 Public Domain
For advanced stages of head and neck cancer, one of the best treatments is so aggressive that it could bring tooth decay, speech loss, constant nausea or all of the above.
That's because the treatment combines two therapies—chemotherapy and radiation—which means double the cancer-killing power, but also double the side effects. A large proportion of the 63,000 Americans diagnosed with head and neck cancer each year are ineligible for this treatment because they are too old or too sick, but most don't know they have the cancer until after the age of 50.
Purdue University researchers in collaboration with the Indiana University School of Medicine have created a new chemoradiotherapy formulation that they predict should be even more effective than what is available commercially. The formulation shouldn't produce side effects because all its toxins stay within tumors, rather than leaking into the bloodstream and harming the whole body.
The study, which appears in a recent issue of the Journal of Controlled Release, shows that the formulation is successful in cancer cell culture experiments, in-vivo animal models and mathematical simulations of patient data. Next, the researchers intend to test the formulation in dogs that naturally have head and neck cancer.
"All previous commercial formulations are not optimized for releasing directly at the tumor under radiation," said You-Yeon Won, a professor of chemical engineering at Purdue, whose lab focuses on improving drug and gene delivery in the body.
"Our formulation has more control and could also be applied to any type of solid tumor, such as those in the breast, prostate, lungs or liver," he said.
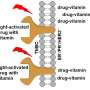
The formulation, designed to be delivered to a tumor via a long syringe needle, is a capsule containing a chemotherapy drug and nanoparticles—tiny compounds that sensitize cancer cells to radiation, making them easier to kill.
Once injected into the body and activated by X-ray radiation, the nanoparticles produce UV light, cracking open the capsule coating so that the chemotherapy drug rapidly releases to a tumor. The coating is biocompatible and approved by the FDA.
Here's the edge: The drug stays in the tumor at least a month—far longer than the minimal therapeutic threshold—and its concentration outside of the tumor stays well below a toxic threshold. Since the drug toxins aren't leaking out of the tumor much, they aren't damaging other cells, which means that there would be virtually no side effects.
The radiation also isn't tearing through other tissue, causing problems like tooth decay, because the nanoparticles keep it targeted at cancer cells.
"We've developed the first-in-kind chemoradiotherapy formulation that can release drugs in response to X-ray radiation. Only under radiation does it start releasing the drug," Won said.
The formulation has been patented via the Purdue Research Foundation Office of Technology Commercialization.
More information: Rahul Misra et al, Radioluminescent nanoparticles for radiation-controlled release of drugs, Journal of Controlled Release (2019). DOI: 10.1016/j.jconrel.2019.04.033
Journal information: Journal of Controlled Release
Provided by Purdue University