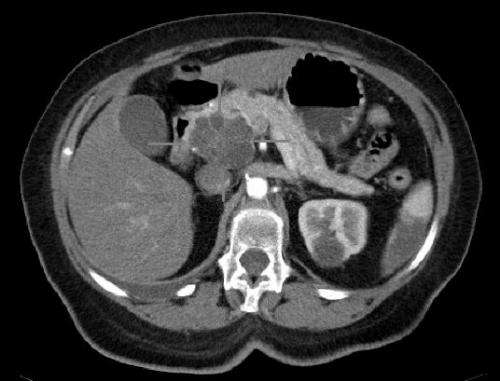
Axial CT image with i.v. contrast. Macrocystic adenocarcinoma of the pancreatic head. Credit: public domain
A new pilot study demonstrated the feasibility of using molecular tumor markers as the basis for selecting the chemotherapeutic agents to use in patients with metastatic pancreatic cancer. Based on these promising results a larger phase II clinical trial has been initiated using molecular biomarkers to guide the choice of second-line therapies. The design, results, and implications of the initial pilot study are presented in Journal of Pancreatic Cancer.
"A Pilot Trial of Molecularly Tailored Therapy for Patients with Metastatic Pancreatic Ductal Adenocarcinoma" was coauthored by Michael Pishvaian, MD, Ph.D., Georgetown University, Washington, DC and colleagues from Wayne State University, Detroit, MI, Georgetown University, and Thomas Jefferson University, Philadelphia, PA.
The researchers designed a composite treatment algorithm based on three established predictive markers of response to chemotherapy. They tested tumor biopsy samples for the presence of these markers and, based on the results, assigned the patients to treatment with two chemotherapeutic agents most likely to elicit a response. The researchers reported promising progression-free survival and overall survival, with a partial response seen in 28% of patients and stable disease in 50% on completion of the study.
Journal of Pancreatic Cancer Editor-in-Chief Charles J. Yeo, MD, Department of Surgery, Thomas Jefferson University, states: "This important study solidifies the potential for a precision medicine approach to pancreatic cancer."
More information: Anteneh A. Tesfaye et al, A Pilot Trial of Molecularly Tailored Therapy for Patients with Metastatic Pancreatic Ductal Adenocarcinoma, Journal of Pancreatic Cancer (2019). DOI: 10.1089/pancan.2019.0003
Provided by Mary Ann Liebert, Inc