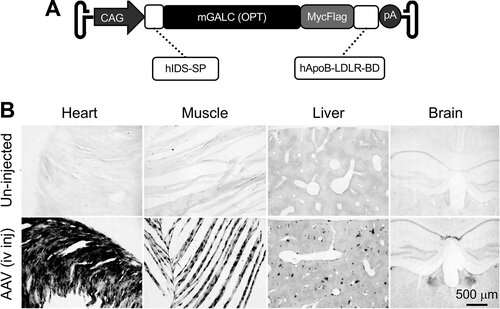
Figure 1. AAV9 galactosylceramidase vector engineering. (A) Schematic outline of the vector. CAG, cytomegalovirus early enhancer/chicken β-actin promoter; hIDS-SP, the coding sequence of the signal peptide of the human iduronate-2-sulfatase gene; mGALC, the codon-optimized mouse galactosylceramidase gene coding sequence; hApoB-LDLR-BD, the coding sequence of the low-density lipoprotein receptor binding domain of the human apolipoprotein B gene; pA, polyadenylation signal. (B) Representative galactosylceramidase enzymatic staining photomicrographs from a 3-month-old untreated mouse (top panels) and a 3-month-old mouse that had received intravenous injection of the AAV9 galactosylceramidase vector at 2 days of age (bottom panels). Systemic injection resulted in widespread galactosylceramidase expression in multiple organs. AAV9, adeno-associated virus serotype 9. https://doi.org/10.1089/hum.2019.008
An optimized and newly engineered form of the adeno-associated vector 9 (AAV9) vector used to deliver the galactosylceramidase gene to a mouse model of the inherited neurogenerative and rapidly fatal form of Krabbe disease improved clinical symptoms and prolonged median survival by 275 percent. Two-day old mice treated with a single injection of the systemic gene therapy had a significant increase in median life span to 150 days, compared to 41 days for the untreated mice, as reported in the study published in Human Gene Therapy.
The article, titled "An Engineered Galactosylceramidase Construct Improves AAV Gene Therapy for Krabbe Disease in Twitcher Mice," was coauthored by Dongsheng Duan, Steven LeVine, and colleagues from the University of Missouri (Columbia), University of Missouri School of Medicine and College of Veterinary Medicine, and University of Kansas Medical Center (Kansas City).
The researchers describe the construction and characterization of the specially engineered AAV9 vector, built on the codon-optimized mouse galactosylceramidase coding sequence, and designed for improved protein delivery to the central nervous system and enhanced secretion of the galactosylceramidase enzyme. At 5 weeks of age, the treated mice had better body weight and motor function than the untreated mice. The longest-lived treated mouse survived to 180 days.
"This work shows dramatic in vivo proof-of-principle, indicating a robust therapeutic effect from this optimized vector," says Editor-in-Chief Terence R. Flotte, MD.
More information: Xiufang Pan et al. An Engineered Galactosylceramidase Construct Improves AAV Gene Therapy for Krabbe Disease in Twitcher Mice, Human Gene Therapy (2019). DOI: 10.1089/hum.2019.008. www.liebertpub.com/doi/10.1089/hum.2019.008
Journal information: Human Gene Therapy
Provided by Mary Ann Liebert, Inc