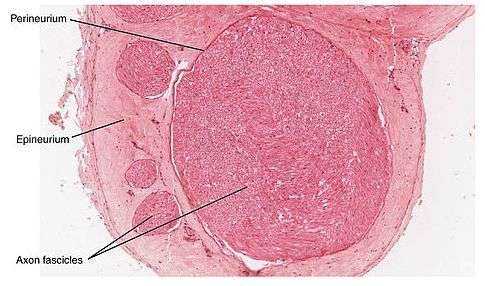
Cross-section of a nerve. Credit: OpenStax College/Wikipedia
Charcot-Marie-Tooth (CMT) disease is the most common inherited disorder of the peripheral nerves in humans, affecting 1 in every 2,500 people. There is no cure for the disease, which causes severe disability due to disruptions in myelin, the protective insulation that covers nerve fibers.
The most common form of CMT is associated with gene duplication and point mutations in the gene for peripheral myelin protein 22 (PMP22). Little is known about the functional role of PMP22 in myelination, however, or how genetic changes in the protein cause disease.
Now Carlos Vanoye, Ph.D., at Northwestern University Feinberg School of Medicine, Vanderbilt University's Bruce Carter, Ph.D., and colleagues provide evidence that PMP22 regulates calcium homeostasis in the Schwann cells that wrap around nerve fibers to form the myelin sheath.
Because high levels of intracellular calcium ions in Schwann cells can induce demyelination, these results, reported August 9 in the Journal of Biological Chemistry, provide novel insights into how genetically altered PMP22 contributes to the pathogenesis of CMT.
More information: Carlos G. Vanoye et al. Peripheral myelin protein 22 modulates store-operated calcium channel activity, providing insights into Charcot-Marie-Tooth disease etiology, Journal of Biological Chemistry (2019). DOI: 10.1074/jbc.RA118.006248
Journal information: Journal of Biological Chemistry
Provided by Vanderbilt University