Study identifies protein responsible for mechanism behind bone loss

Researchers from the UCLA School of Dentistry have provided insight into how the mechanical process of bone loss works and have also identified a protein that is responsible for recycling of the cells that can also promote bone loss. The team showed that by eliminating a key protein responsible for the activation of bone loss, there is the potential to control the level of bone loss a person would develop.
Osteoporosis is a bone loss condition that can cause serious medical problems. About 10 million Americans have received diagnoses of osteoporosis and an additional 44 million have low bone density. The main culprits in bone loss are osteoclasts, which are the cells that break bone down and work in tandem with osteoblasts, which are cells that are responsible for bone formation, to maintain bone volume. But when those osteoclasts over-perform their function, the results are losses in bone density and bone.
It is well known that RANKL, a protein that affects the immune system and controls bone regeneration, plays a critical role in osteoclasts being activated, but the actual mechanism by which the protein regulates an osteoclast's pathway toward bone loss is not completely understood. In this study, the researchers set out to better understand RANKL's role in directing osteoclasts to promote bone loss and whether there could be a way to control the pathway toward bone regeneration or bone growth.
The researchers focused their study on the autophagy, or cellular regeneration process, in osteoclasts in order to figure out why RANKL promotes bone loss. It is well-known that autophagy becomes activated primarily during cellular stress, such as starvation. However, there is growing evidence that shows autophagy can be activated through signaling pathways and that cellular functions could be controlled.
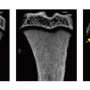
They started by examining Beclin1, an indispensable protein for autophagy initiation, in combination with RANKL-activated cells, both in in vitro cell work and in in vivo (in the living organism) studies of mice. The team evaluated several models of Beclin1-enhanced combinations and came to the conclusion that an overexpression of the Beclin1 protein was directly tied to cells changing into osteoclasts. They went one step further by developing a mouse model where they eliminated Beclin1 in osteoclasts and the results were thicker cortical bone, which is bone tissue that's denser than other types and accounts for up to 80% of the weight of a human's skeleton.
According to the National Osteoporosis Foundation, experts predict that by 2025, osteoporosis will be responsible for 3 million fractures, resulting in $25.3 billion in health care-related costs. The researchers' method of targeting autophagy in osteoclasts would be a valuable strategic method to prevent pathologic, or disease-caused, bone loss and could lead to improved therapies for osteoporosis and osteonecrosis of the jaw, a condition where bone cells in the jaw break down or die.
More information: Atsushi Arai et al. Beclin1 Modulates Bone Homeostasis by Regulating Osteoclast and Chondrocyte Differentiation, Journal of Bone and Mineral Research (2019). DOI: 10.1002/jbmr.3756