Brain tumors form synapses with healthy neurons, study finds
Scientists at the Stanford University School of Medicine have shown for the first time that severe brain cancers integrate into the brain's wiring.
The tumors, called high-grade gliomas, form synapses that hijack electrical signals from healthy nerve cells to drive their own growth. Experiments demonstrated that interrupting these signals with an existing anti-epilepsy drug greatly reduced the cancers' growth in human tumors in mice, providing the first evidence for a possible new way to treat gliomas.
A paper describing the findings will be published online Sept. 18 in Nature.
"One of the most lethal aspects of high-grade gliomas is that the cancer cells diffusely invade normal brain tissue so that the tumor and the healthy brain tissue are knitted together," said senior author Michelle Monje, MD, Ph.D., associate professor of neurology and neurological sciences. The discovery helps explain why gliomas are so intractable, she added. "This is such an insidious group of tumors. They're actually integrating into the brain."
The study's lead author is postdoctoral scholar Humsa Venkatesh, Ph.D.
Discovering that tumors wire themselves into the brain was "unsettling," Monje said. Still, she said she is optimistic about what the knowledge means for glioma patients. Several drugs already exist for treating electrical-signaling disorders such as epilepsy, and these may prove useful for gliomas, she said. "There is real hopefulness to this discovery," she said. "We've been missing this entire aspect of the disease. Now we have a whole new avenue to explore, one that could complement existing therapeutic approaches."
How the tumors grow
High-grade gliomas form synapses with healthy neurons that transmit electrical signals to the cancerous tissue, the study found. The tumors also contain cell-to-cell electrical connections known as gap junctions. Together, the two types of connections allow electrical signals from healthy nerve cells to be conducted into and amplified within the tumors.
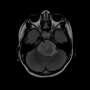
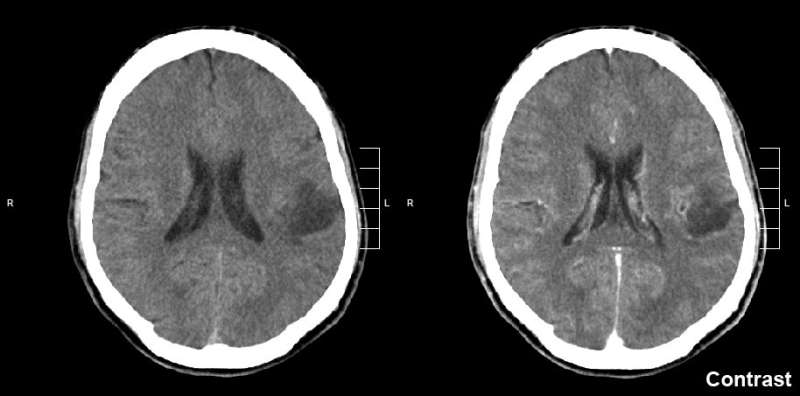
High-grade gliomas include glioblastoma, a brain tumor seen in adults that has a five-year survival rate of 5%; diffuse intrinsic pontine glioma, a pediatric brain tumor with a five-year survival rate below 1%; and other diagnoses such as pediatric glioblastoma and diffuse midline gliomas occurring in the spinal cord and thalamus. Studies published by Monje's team in 2015 and 2017 indicated that high-grade gliomas use normal brain activity to drive their growth.
To learn how this worked, the scientists first analyzed the gene expression of thousands of individual cancer cells biopsied from newly diagnosed glioma patients. The cancer cells strongly increased the expression of genes involved in forming synapses.
The researchers then used electron microscopy, a technique that can reveal tiny details of cell anatomy, to show that structures that look like synapses exist between neurons and glioma cells. To confirm that these synapses indeed connect healthy neurons and malignant glioma cells, the scientists studied mice with cells from human gliomas implanted in their brains. After the glioma tumors had become established, the researchers used antibodies that bound to fluorescent markers expressed by the cancer cells to confirm that synapses go into malignant cells. "We saw very clear neuron-to-glioma synaptic structures," Monje said.
Using brain tissue from mice with human gliomas, the researchers measured the transmission of electrical signals into and through the tumors. They recorded two types of electrical signals: brief signals lasting four to five milliseconds, which are transmitted across a synaptic junction from a healthy neuron to a cancer cell by way of neurotransmitter molecules; and sustained electrical signals lasting one to two seconds that reflect electrical current propagated by a flux of potassium ions across the tumor cells' membranes. The potassium currents are caused by signals from neurons and are amplified by gap junctions that connect the cancer cells in an electrically coupled network.
The scientists also conducted experiments using a dye to visualize the gap-junction-connected cells, and used drugs capable of blocking gap junctions to confirm that this type of junction existed between the tumor cells and mediated their electrical coupling. Further experiments measuring changes in calcium levels confirmed that the tumor cells are electrically coupled via gap junctions.
"The live calcium imaging made it strikingly clear that this cancer is an electrically active tissue," said Venkatesh, the lead author. "It was startling to see that in cancer tissue."
The researchers showed that about 5-10% of glioma cells receive synaptic signals, and about 40% exhibit prolonged potassium currents that are amplified by gap junction interconnections such that half of all tumor cells have some type of electrical response to signals from healthy neurons.
Possible drug therapies
In humans who were having the electrical activity in their brains measured before surgery to remove glioblastoma tumors, and in mice with human gliomas, the researchers saw hyper-excitability of healthy neurons near the tumors, a finding that could help explain why human glioma patients are prone to seizures.
Using optogenetic techniques, which relied on laser light to activate the cancer cells in mice implanted with human gliomas, the researchers demonstrated that increasing electrical signals into the tumors caused more tumor growth. Proliferation of the tumors was largely prevented when glioma cells expressed a gene that blocked transmission of the electrical signals.
Existing drugs that block electrical currents also reduced growth of high-grade gliomas, the research found. A seizure medication called perampanel, which blocks activity of neurotransmitter receptors on the receiving end of a synapse, reduced proliferation of pediatric gliomas implanted into mice by 50%. Meclofenamate, a drug that blocks the action of gap junctions, resulted in a similar decrease in tumor proliferation.
Monje's team plans to continue investigating whether blocking electrical signaling within tumors could help people with high-grade gliomas. "It's a really hopeful new direction, and as a clinician I'm quite excited about it," she said.
More information: Electrical and synaptic integration of glioma into neural circuits, Nature (2019). DOI: 10.1038/s41586-019-1563-y , nature.com/articles/s41586-019-1563-y