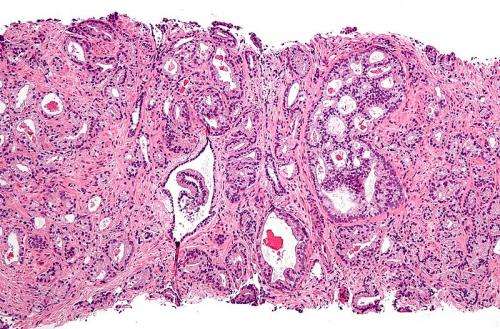
Micrograph showing prostatic acinar adenocarcinoma (the most common form of prostate cancer) Credit: Wikipedia, CC BY-SA 3.0
PTEN, a tumor suppressor gene mutated in approximately 20% of primary prostate cancers, and in as many as 50% of androgen deprivation-resistant prostate cancers, relies on another gene, ARID4B, to function. These findings were published by George Washington University (GW) Cancer Center researchers in Nature Communications. This discovery provides a potential therapeutic target for prostate cancers carrying the common PTEN mutation.
"Loss of the tumor suppressor PTEN due to mutation or deletion not only is frequent in human prostate cancer, but also plays a large role in other cancers. We wanted to find out more about PTEN, and other genes it might rely on, to offer new treatment options for those with the PTEN mutation," said Ray-Chang Wu, Ph.D., associate professor of biochemistry and molecular medicine at the GW School of Medicine and Health Sciences. "We discovered that PTEN has an important connection to the gene ARID4B, which offers a new therapeutic target for treatment."
Wu, his co-author Mei-Yi Wu, Ph.D., associate professor of medicine at the GW School of Medicine and Health Sciences, and other members of the research team at the GW Cancer Center examined data from several prostate cancer cohorts and made an interesting observation: cancers which contain PTEN mutations almost always retain ARID4B. One function of the gene ARID4B includes remodeling the chromatin that makes up the chromosome. This "mutually exclusive" pattern between PTEN and ARID4B offers the team the first clue as to its potential importance in prostate cancer.
The research further found that suppression of ARID4B expression in cancer cells with PTEN mutation significantly inhibits cancer cell growth and increases cell death. In comparison, less pronounced effects were observed when cancer cells that contain functional PTEN were used, suggesting a dependence on ARID4B by PTEN-deficient prostate cancer. Importantly, the team is able to recapitulate these findings using the PTEN-deleted prostate cancer mouse models. As expected, deletion of PTEN alone in mice leads to development of prostate cancer. In stark contrast, mice with deletion of both PTEN and ARID4B do not develop tumors. Collectively, these results led the team to conclude that PTEN function depends on the presence of ARID4B and identify ARID4B as a potential therapeutic target in prostate cancer, given loss of the PTEN gene. More research is needed to develop methods to target ARID4B.
The article, "Identification of the PTEN-ARID4B-PI3K pathway reveals the dependency on ARID4B by PTEN-deficient prostate cancer," is published in Nature Communications.
More information: Ray-Chang Wu et al, Identification of the PTEN-ARID4B-PI3K pathway reveals the dependency on ARID4B by PTEN-deficient prostate cancer, Nature Communications (2019). DOI: 10.1038/s41467-019-12184-8
Journal information: Nature Communications
Provided by George Washington University