Gene mutation in the chloride channel triggers hyperaldosteronism
Hyperaldosteronism is a disease primarily affecting the adrenal glands that leads to high blood pressure due to overproduction of the hormone aldosterone and that may also damage the kidneys. It has only recently emerged that several patients with this condition harbor a mutation in the gene for the ClC-2 chloride channel. Researchers from the Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP) and the Max Delbrück Center (MDC) in Berlin now generated a new in-vivo mouse model to show how mutations in this channel cause the disease. In addition to deciphering the disease mechanism step by step, the researchers have paved the way for further research into the complex clinical picture. Their results have just been published in the journal Nature Communications.
The steroid hormone aldosterone, in concert with other mechanisms, controls our blood pressure. It is secreted by the adrenal glands and regulates the water and salt balance in the body. Adrenal glands in patients affected by hyperaldosteronism produce excessive amounts of aldosterone, which leads to excessive sodium retention which in turn increases the excretion of potassium. In the end, this leads to abnormally high blood pressure, "arterial hypertension." The combination of high aldosterone concentration and high blood pressure often results additionally in kidney damage.
The pathological mechanisms of the disease, also known as Conn syndrome, remained incompletely understood. In 2018, Paris-based scientists around Dr. Maria-Christina Zennaro teamed up with colleagues from FMP and MDC in Berlin, as well as other scientists in Germany and the U.S. around Prof. Ute Scholl from the Berlin Institute of Health (BIH) and the Charité – Universitätsmedizin Berlin, and found mutations in the ClC-2 chloride channel in patients affected by this syndrome. To date, a total of six different mutations have been described (published in Nature Genetics 2018). However, the pathway leading from the mutations to aldosterone overproduction had remained unclear—until researchers from FMP and MDC generated and analyzed a specific mouse model.
Proven causality between mutation and disease
The team, led by Prof. Thomas Jentsch—a pioneer who discovered the first chloride channel family, including ClC-2, almost three decades ago, initially investigated all known aldosteronism-causing ClC-2 mutations in vitro. They found that all these mutations drastically increased the flow of chloride through the channel.
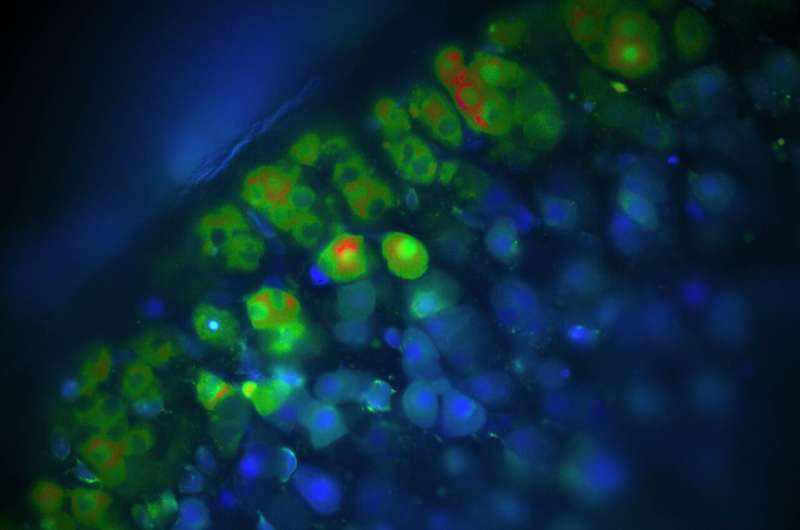
To examine the hypothesis that increased chloride flow through ClC-2 causes hyperaldosteronism, the researchers then developed a mouse model in which ClC-2 was activated by an "artificial" mutation that had not been reported for patients. The genetically modified mice exhibited enormously increased chloride currents in aldosterone-secreting cells, which indirectly led to a large increase in aldosterone concentration in the blood of those rodents. Just like in patients, this resulted in abnormally elevated blood pressure and secondarily reduced activity of renin, a hormone that normally boosts aldosterone production. In addition to proving that an increase in Cl- currents in adrenal gland cells leads to hyperaldosteronism, the researchers investigated the pathological pathway in great detail.
Chloride channel continuously open
"We have seen how the channel is constantly open due to these mutations, which greatly changes the electrical voltage across the membrane of the hormone-producing cell. This leads to an influx of calcium, which, in turn, causes overproduction of aldosterone," explains Dr. Corinna Göppner, who, together with Dr. Ian Orozco, is the first author of the article.
The best model to explore hyperaldosteronism
The mouse model developed in Berlin Buch is the first in-vivo model that faithfully models all symptoms of the disease. Therefore, it is the perfect model to investigate the pathological mechanisms of hyperaldosteronism and to identify secondary effects such as long-term damage. The researchers were already able to detect slight kidney damage, but they hope more effects will be discovered: "At the moment, we assume that a constitutively open chloride channel could also impact other organs," says group leader Prof. Thomas Jentsch. Unfortunately, this is still a grey area in medicine, although the long-term consequences are highly relevant for patients. "Our mouse model can definitely help to clarify the situation. This reaffirms once again, how relevant basic research can be for clinical applications."
More information: Corinna Göppner et al. Pathogenesis of hypertension in a mouse model for human CLCN2 related hyperaldosteronism, Nature Communications (2019). DOI: 10.1038/s41467-019-12113-9