Subunit contribution to NMDA receptor hypofunction, redox sensitivity of hippocampal synaptic transmission during aging
Researchers examined the contribution of N-methyl-D-aspartate receptor subunits in the redox-mediated decline in NMDAR function during aging.
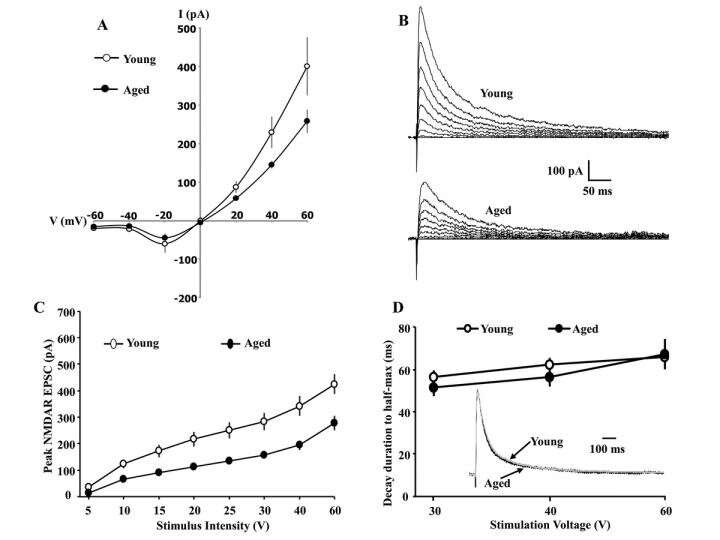
Glu N2A and Glu N2B selective antagonists decreased peak NMDAR currents to a similar extent in young and aged animals, indicating that a shift in diheteromeric Glu N2 subunits does not underlie the age-related decrease in the NMDAR synaptic function.
The results indicate that activity-dependent NMDAR synaptic plasticity is suppressed by redox-mediated inhibition of Ca MKII activation during aging.
The redox regulation of NMDARs represents a suppression of a metaplasticity mechanism, which can disrupt synaptic plasticity and cognition associated with neurological or psychiatric diseases, and aging.
Dr. Ashok Kumar and Dr. Thomas C. Foster said, "The function of N-methyl-D-aspartate receptors have a profound influence on synaptic plasticity, cognition, psychiatric diseases, and the connectivity of neural networks."
The results point to a redox sensitive mechanism in mediating the well-characterized decrease in the CA3-CA1 NMDAR synaptic response of older-memory impaired animals, and suggests that redox regulation of NMDARs influences synaptic plasticity during aging.
NMDARs are heterotetramers and previous research has focused on diheteromeric NMDARs with two Glu N1 subunits and two identical Glu N2 subunits, either Glu N2A or Glu N2B. Over the course of development, many brain regions exhibit an increase in the decay rate of NMDAR synaptic responses resulting from an increased contribution of Glu N2A to NMDAR responses.
If redox regulation is acting through NMDAR plasticity involving Glu N2B, DTT application should increase the Glu N2B contribution to the synaptic response.
The current study, recorded synaptically evoked excitatory postsynaptic currents from CA1 hippocampal pyramidal neurons and field excitatory postsynaptic potentials from CA3-CA1 synapses, and examined the contribution of Glu N2A and Glu N2B subunits to the decline in NMDAR synaptic function during aging, and the DTT-induced enhancement of NMDAR-mediated synaptic transmission.
The Kumar/Foster Research team concluded, "Redox mediated NMDAR hypofunction can act as metaplasticity mechanism, regulating synaptic modifiability required for synaptic networks that underlie cognition."
More information: Ashok Kumar et al, Subunit contribution to NMDA receptor hypofunction and redox sensitivity of hippocampal synaptic transmission during aging, Aging (2019). DOI: 10.18632/aging.102108