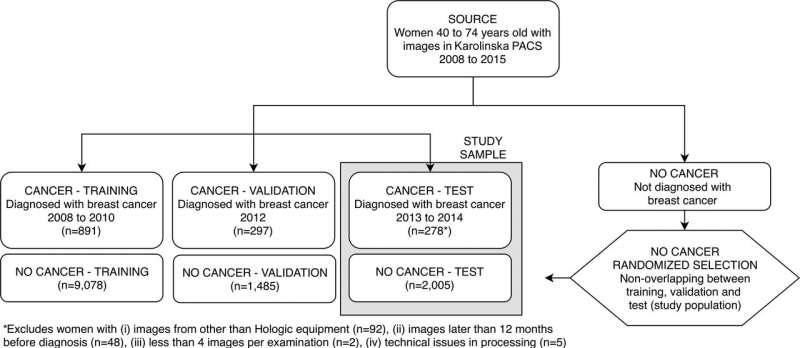
Patient inclusion flowchart shows selection of women in the training and validation samples used for deep neural network development, as well as in the test sample (current study sample). Exclusions are detailed in the footnote. PACS = picture archiving and communication system. Credit: Radiological Society of North America
A sophisticated type of artificial intelligence (AI) can outperform existing models at predicting which women are at future risk of breast cancer, according to a study published in the journal Radiology.
Most existing breast cancer screening programs are based on mammography at similar time intervals—typically, annually or every two years—for all women. This "one size fits all" approach is not optimized for cancer detection on an individual level and may hamper the effectiveness of screening programs.
"Risk prediction is an important building block of an individually adapted screening policy," said study lead author Karin Dembrower, M.D., breast radiologist and Ph.D. candidate from the Karolinska Institute in Stockholm, Sweden. "Effective risk prediction can improve attendance and confidence in screening programs."
High breast density, or a greater amount of glandular and connective tissue compared to fat, is considered a risk factor for cancer. While density may be incorporated into risk assessment, current prediction models may fail to fully take advantage of all the rich information found in mammograms. This information has the potential to identify women who would benefit from additional screening with MRI.
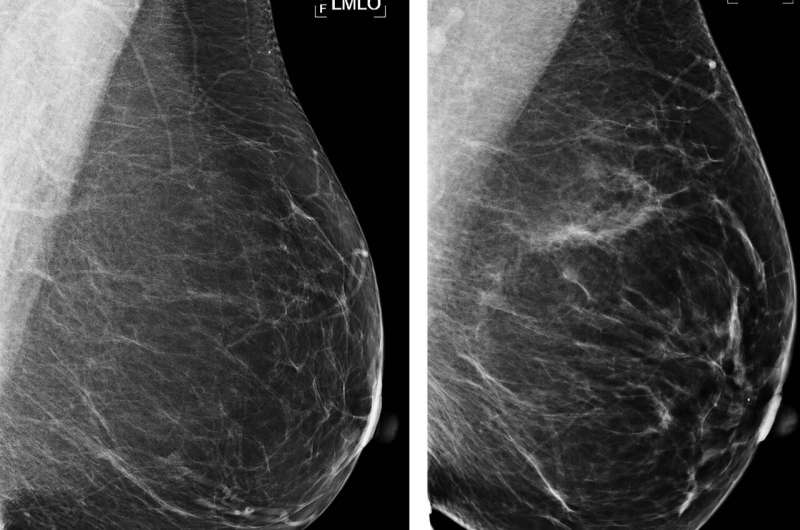
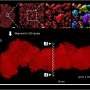
Examples of mammograms with concordance between deep learning (DL) risk score and outcome of breast cancer (true predictions). All images are mediolateral oblique views of left breast. (a) Mammogram in 55-year-old woman with low DL risk score (0.05) who was not diagnosed with breast cancer (ie, true-negative prediction). (b) Mammogram in 47-year-old woman with low DL risk score (0.06) who was not diagnosed with breast cancer (ie, true-negative prediction). (c) Mammogram in 56-year-old woman with high DL risk score (0.30) who received a diagnosis of breast cancer 5.1 years after the examination (ie, true-positive prediction). (d) Mammogram in 57-year-old woman with high DL risk score (0.30) who received a diagnosis of breast cancer 5.0 years after the examination (ie, true-positive prediction). LMLO = left mediolateral oblique. Credit: Radiological Society of North America
Dr. Dembrower and colleagues developed a risk model that relies on a deep neural network, a type of AI that can extract vast amounts of information from mammographic images. It has inherent advantages over other methods like visual assessment of mammographic density by the radiologist that may not be able to capture all risk-relevant information in the image.
The new model was developed and trained on mammograms from cases diagnosed between 2008 and 2012 and then studied on more than 2,000 women ages 40 to 74 who had undergone mammography in the Karolinska University Hospital system. Of the 2,283 women in the study, 278 were later diagnosed with breast cancer.
The deep neural network showed a higher risk association for breast cancer compared to the best mammographic density model. The false negative rate—the rate at which women who were not categorized as high-risk were later diagnosed with breast cancer—was lower for the deep neural network than for the best mammographic density model.
"The deep neural network overall was better than density-based models," Dr. Dembrower said. "And it did not have the same bias as the density-based model. Its predictive accuracy was not negatively affected by more aggressive cancer subtypes."
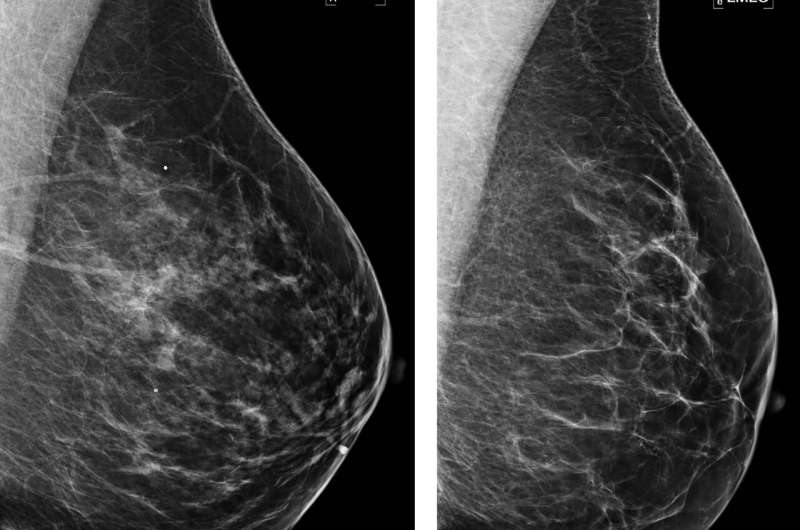
Examples of mammograms with discordance between deep learning (DL) risk score and outcome of breast cancer (false predictions). All images are mediolateral oblique views of left breast. (a) Mammogram in 44-year-old woman with low DL risk score (0.05) who received a diagnosis of breast cancer 4.7 years after the examination (ie, false-negative prediction). (b) Mammogram in 40-year-old woman with low DL risk score (0.07) who received a diagnosis of breast cancer 4.1 years after the examination (ie, false-negative prediction). (c) Mammogram in 65-year-old woman with high DL risk score (0.53) who was not diagnosed with breast cancer (ie, false-positive prediction). (d) Mammogram in 65-year-old woman with high DL risk score (0.48) who was not diagnosed with breast cancer (ie, false-positive prediction). LMLO = left mediolateral oblique. Credit: Radiological Society of North America
The study findings support a future role for AI in breast cancer risk assessment.
"We are not reporting mammographic density currently," Dr. Dembrower said. "In the introduction of individually adapted screening, we use deep learning networks trained to predict cancer rather than taking the indirect route that density offers."
As an additional benefit, the AI approach can continually be improved with exposure to more high-quality data sets.
"Our deep learning experts at the Royal Institute of Technology in Stockholm are working on an update to the model," Dr. Dembrower said. "After that, we aim to test the model clinically next year by offering MRI to the women who stand to benefit the most."
More information: Comparison of a Deep Learning Risk Score and Standard Mammographic Density Score for Breast Cancer Risk Prediction, Radiology, 2019.
Journal information: Radiology
Provided by Radiological Society of North America