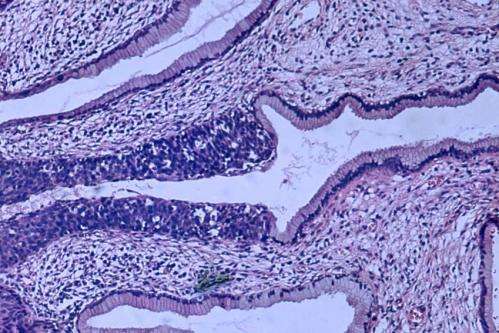
High grade dysplasia (carcinoma in situ) in the uterine cervix. The abnormal epithelium is extending into a mucus gland to the left of centre. This disease can progress to invasive cancer (squamous cell carcinoma) of the cervix. Credit: Haymanj/public domain
Results from the NRG Oncology phase I clinical trial NRG-GOG 9929 show that using the immunotherapy drug ipilimumab after chemoradiotherapy (CRT) is tolerated in the curative treatment of women with lymph node-positive cervical cancer. The maximum tolerated dose of ipilimumab was determined to be 10 mg/kg. These results are published in JAMA Oncology and was be highlighted at a gynecologic session during the American Society for Radiation Oncology's (ASTRO) Annual Meeting in September 2019.
NRG-GOG 9929 is the first trial to describe the safety of immunotherapy following CRT in curative cervical cancer. Currently, many women with node-positive cervical cancer who are treated with CRT will recur. Therein lies the need to develop a safe and effective therapeutic strategy, such as the use of immunotherapy, to improve outcomes for women with this type of cancer. NRG-GOG 9929 was designed to primarily determine the safety and proper dosage of ipilimumab (immunotherapy) following the standard CRT treatment; however, a secondary endpoint included in the trial was measuring survival outcomes.
Twenty-one patients on NRG-GOG 9929 received CRT followed by ipilimumab (immunotherapy). Patients received one of two potential dose levels, 3mg/kg (DL1) and 10mg/kg (DL2), in order to determine the maximum dose of immunotherapy. Only two patients experienced self-limited grade 3 toxicities. The 12-month progression free survival rate was 81 percent and the 12-month overall survival rate was 90 percent. All patients completed CRT, 86 percent of women completed four cycles of ipilimumab, and 14 percent of women completed two cycles of ipilimumab. PD-1 expression increased with CRT and was sustained with immunotherapy.
"Future researchers should consider the findings of this prospective phase 1 trial when developing clinical trials for women with node-positive cervical cancer. We need to have more studies and investigations that will help to identify therapeutic targets to improve outcomes in this patient population," stated Jyoti S. Mayadev, MD, of University of California San Diego School of Medicine and lead author of NRG-GOG 9929.
More information: Jyoti S. Mayadev et al, Sequential Ipilimumab After Chemoradiotherapy in Curative-Intent Treatment of Patients With Node-Positive Cervical Cancer, JAMA Oncology (2019). DOI: 10.1001/jamaoncol.2019.3857
Journal information: JAMA Oncology
Provided by NRG Oncology