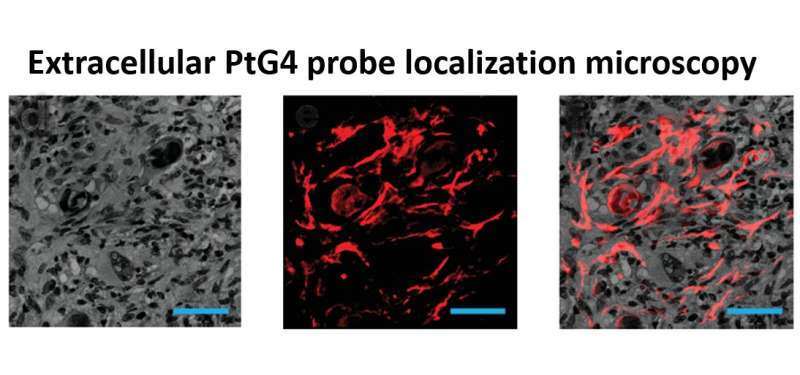
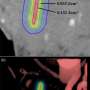
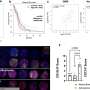
An oxygen map image recovered from a mouse undergoing radiation therapy. The luminescent oxygen probe PtG4 is injected during the week of radiation treatment and localizes between the cells of the tumor as illustrated by microscopy (red). Credit: Brian Pogue, PhD
Oxygen in cancer tumors is known to be a major factor that helps radiation therapy be successful. Hypoxia, or starvation of oxygen, in solid tumors is also thought to be an important factor in resistance to therapy. However, it is difficult to monitor tumor oxygenation without invasive sampling of oxygen distributions throughout the tissue, or without averaging across the whole tumor, whereas oxygen is highly heterogenous within a tumor.
A research team at Dartmouth's and Dartmouth-Hitchcock's Norris Cotton Cancer Center led by Brian Pogue, Ph.D., has developed the first non-invasive way to directly monitor oxygen distributions within the tumor right at the time when radiation therapy is happening. With injection of an oxygen probe drug, PtG4, they are able to image the distribution of oxygen from within the tumor. The method measures the luminescence lifetimes of PtG4 while it is excited by the Cherenkov light emitted by the radiation therapy. The drug, PtG4, stays in the tumor for at least a week, and works for imaging repeatedly.
"The imaging is all done without any additional radiation, simply by using a camera to monitor the emissions during radiotherapy treatment," explains Pogue. "Following two tumor lines, one which is known to be responsive to radiation and one which is known to be resistant, we could see differences in the oxygenation of the tumor which are reflective of their differences in response." The team's findings, "Tissue pO2 Distributions in Xenograft Tumors Dynamically Imaged by Cherenkov-Excited Phosphorescence during Fractionated Radiation Therapy," are newly published in Nature Communications, by lead author, Xu Cao.
Pogue's team is able to capture oxygenation imaging through special technology. "We have a unique set of time-gated cameras in our radiation therapy department that were designed for Cherenkov-based radiation dosimetry, but we have used them for this additional purpose of monitoring oxygen in the tumors under treatment," says Pogue. "So access to these specialized Cherenkov cameras made the measurements possible." Pogue's team also collaborated with Professor Sergei Vinogradov and his team at the University of Pennsylvania Perelman School of Medicine, who produced the PtG4 and supported the work with drug characterization and co-supervision of the study.
Pogue hopes to develop this tumor monitoring ability into a useful clinical aid used to track tumor response to radiation therapy, especially tumors that are known to be hypoxic. Having such information available at the time of treatment could be helpful in influencing treatment decisions such as giving a radiation boost where needed. "When a patient gets radiation therapy, the treatment should be designed to directly utilize as much information about the patient's tumor as possible," says Pogue. "Today, we use the shape of the tumor and the tissue around it. But, we need to also think about using measurements of the tumor metabolism because this affects the success of treatment as well. Future radiation therapy treatments should ideally incorporate metabolic features such as oxygenation of the tumor when the treatment is planned or delivered."
The next steps toward this future are already underway. Pogue's team is looking to characterize how small of a region they can track the oxygenation from, and how fast they can take measurements. "Our goal is to produce oxygen images at video rate, with a spatial resolution that allows us to see radiobiologically relevant hypoxia nodules in the tumor of humans," explains Pogue.
More information: Xu Cao et al, Tissue pO2 distributions in xenograft tumors dynamically imaged by Cherenkov-excited phosphorescence during fractionated radiation therapy, Nature Communications (2020). DOI: 10.1038/s41467-020-14415-9
Journal information: Nature Communications
Provided by Dartmouth-Hitchcock Medical Center