Cancer cell during cell division. Credit: National Institutes of Health
MYC is a family of three related proteins that are overexpressed in cancer and which contribute to an estimated 100,000 cancer deaths annually in the United States.
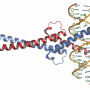
Efforts to block MYC directly have failed. Fortunately, these proteins have an Achilles' heel—a chromosome-binding cofactor called WDR5.
Understanding how MYC interacts with WDR5 and other cofactors could lead to the development of new drugs that can effectively block MYC and stop many cancers in their tracks.
Reporting in the Proceedings of the National Academy of Sciences, William Tansey, Ph.D., and colleagues at Vanderbilt found that disrupting the interaction between MYC and WDR5 in a cancerous growth causes "rapid and comprehensive tumor regression."
"If the MYC–WDR5 connection is to be pursued as a viable therapeutic avenue, we need to know if breaking this connection in the context of an existing model cancer would have an impact on tumor growth," said Tansey, co-leader of the Vanderbilt-Ingram Cancer Center's Genome Maintenance Research Program.
That's what they did, using a laboratory-grown model of Burkitt's lymphoma, a form of non-Hodgkin's lymphoma or immune-cell cancer. "We now know that breaking this connection causes these tumors to disappear," he said.
Tansey is the Ingram Professor of Cancer Research and professor of Cell & Developmental Biology and Biochemistry.
In 2015, in collaboration with Tansey's group, a team led by Stephen Fesik, Ph.D., the Orrin H. Ingram, II Professor of Cancer Research, solved the crystal structure of the MYC-WDR5 interaction. This effort led to their ongoing collaboration to discover drugs that can target MYC through WDR5.
More information: Lance R. Thomas et al. Interaction of the oncoprotein transcription factor MYC with its chromatin cofactor WDR5 is essential for tumor maintenance, Proceedings of the National Academy of Sciences (2019). DOI: 10.1073/pnas.1910391116
Journal information: Proceedings of the National Academy of Sciences
Provided by Vanderbilt University