Uncovering the unusual way aggregates hijack proteins
Utrecht scientists have uncovered how aggregates alter the behavior of Tau protein, known to play a role in Alzheimer's disease. On a molecular level, the researchers have revealed why certain proteins tend to bind to Tau proteins. The key is the unusual way these proteins bind to Tau. The findings are published in Nature Communications.
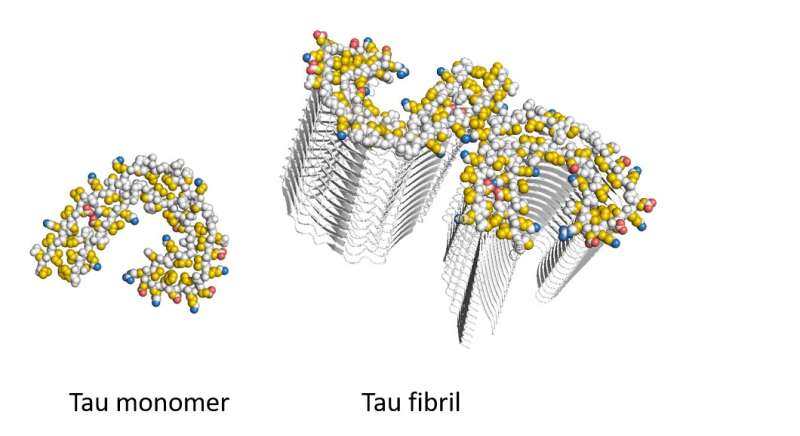
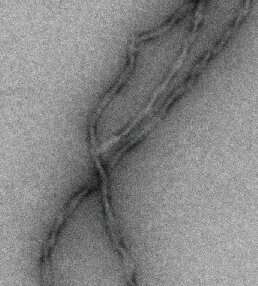
The hallmark of Alzheimer's disease is the death of neurons caused by aggregation of the Tau protein in the brain. Key is the formation of fibrils: long needle-like Tau protein aggregates. It is unclear, however, why these protein aggregations kills neurons. "There is no cure, simply because the molecular basis of the disease is poorly understood," says last author Stefan Rüdiger, of the section Cellular Protein Chemistry at Utrecht University.
The researchers produced aggregates of the Alzheimer's protein Tau and exposed them to a mixture containing all proteins that reside in the brain. "Mass spectrometric analysis revealed, that proteins binding exclusively to Tau aggregates belong to specific classes, known to play a role in Alzheimer's development. Analysis of their composition revealed that they bind via an unusual mechanism to the Tau fibrils, so-called pi-stacking," Rüdiger explains.
Stacking
This pancake-like stacking of Tau fibrils drives binding of protein to the Tau fibrils. In the publication the researchers reveal on a molecular level the mechanism behind the protein aggregation and how this impacts on cellular processes. This is an essential first step for targeting the cause of the disease.
"All diseases caused by protein aggregation—Parkinson's, Huntington's, Alzheimer's—lack fundamental understanding of the disease," says Rüdiger. "It is essential to understand the disease if we want to be able to treat it."
More information: Luca Ferrari et al. Arginine π-stacking drives binding to fibrils of the Alzheimer protein Tau, Nature Communications (2020). DOI: 10.1038/s41467-019-13745-7