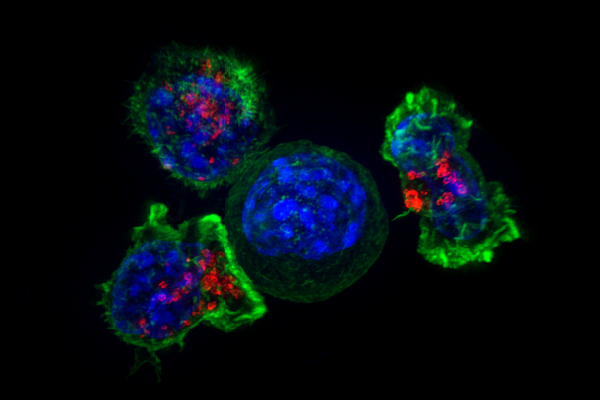
Killer T cells surround a cancer cell. Credit: NIH
A new Scientific Reports paper puts an evolutionary twist on a classic question. Instead of asking why we get cancer, Leonardo Oña of Osnabrück University and Michael Lachmann of the Santa Fe Institute use signaling theory to explore how our bodies have evolved to keep us from getting more cancer.
It isn't obvious why, when any cancer arises, it doesn't very quickly learn to take advantage of the body's own signaling mechanisms for quick growth. After all, unlike an infection, cancers can easily use the body's own chemical language. "Any signal that the body uses, an infection has to evolve to make," says Lachmann. "If a thief wants to unlock your house, they have to figure out how to pick the lock on the door. But cancer cells have the keys to your house. How do you protect against that? How do you protect against an intruder who knows everything you know, and has all the tools and keys you have?" Their answer: You make the keys very costly to use.
Oña and Lachmann's evolutionary model reveals two factors in our cellular architecture that thwart cancer: the expense of manufacturing growth factors ("keys") and the range of benefits delivered to cells nearby. Individual cancer cells are kept in check when there's a high energetic cost for creating growth factors that signal cell growth. To understand the evolutionary dynamics in the model, the authors emphasize the importance of thinking about the competition between a mutant cancerous cell and surrounding cells. When a mutant cell arises and puts out a signal for growth, that signal also provides resources to adjacent, non-mutated cells. Thus, when the benefits are distributed to a radius around the signaling cell, the mutant cells have a hard time out-competing their neighbors and can't get established. The cancer loses the ability to give the signal.
The work represents a novel application of evolutionary biology toward a big-picture understanding of cancer. Oña and Lachmann draw from the late biologist Amos Zahavi's handicap principle, which explains how evolutionary systems are stabilized against "cheaters" when dishonest signals are costlier to produce than the benefit they provide. The male peacock's elaborate tail is the classic example of a costly signal—an unhealthy bird would not have the energetic resources to grow an elaborate tail, and thus could not "fake" a signal of their evolutionary fitness. By the handicap principle, a cancer cell would be analogous to the unhealthy peacock that can't afford to signal for attention.
So how do some cancer cells overcome these evolutionary constraints? The authors point out that their model only addresses the scenario of an individual cancer trying to invade a healthy population. Once a cancer has overcome the odds of extinction and reached a certain critical size, other dynamics prevail.
"Many mechanisms seem to have evolved to prevent cancer—from immune system control, cell death, limits on cell proliferation, to tissue architecture," the authors write. "Our model only studies the reduced chance for invasion."
"Cancer is incredibly complex," Lachmann says, "and our model is relatively simple. Still, we believe it's an important step toward understanding cancer and cancer prevention in evolutionary terms."
More information: Leonardo Oña et al, Signalling architectures can prevent cancer evolution, Scientific Reports (2020). DOI: 10.1038/s41598-020-57494-w
Journal information: Scientific Reports
Provided by Santa Fe Institute