Can low IVF success be reversed with new insights into egg cell aging?

A UNSW researcher explores the biology of infertility, and explains how a recent study in mice contributes to our understanding of reproductive aging, with the aim of improving IVF success rates in humans.
Couples today are increasingly delaying parenthood until later in life, for reasons including career and financial goals. For many, this is pushing up against biological limits to natural fertility: fertility is already declining in the thirties, and the chances of conceiving naturally when you're 19-25 fall by half when you're 30-35, contributing to the social concept of a "ticking clock" for family planning. For couples that have trouble in achieving pregnancy, assisted reproductive technologies such as in vitro fertilisation (IVF) can provide a helping hand, and the demand for this procedure is growing at a massive pace across the world.
While IVF is becoming increasingly widespread, its success is far from guaranteed: in Australia and New Zealand, the success rate of each IVF cycle in women under the age of 30 is only 29%, rapidly dropping to only 16.5% in women between the ages of 35-39, and 5.2% between the ages of 40-44. These low success rates mean that women typically have to undergo repeated rounds of IVF cycles, with couples having to experience the psychological distress and relationship strain of each failed cycle. On top of this, IVF can be financially draining to both patients and the healthcare system: in Australia, Medicare generously subsidises an unlimited number of cycles, regardless of patient age, however patients are often still left with out-of-pocket costs in the thousands of dollars per cycle.
Egg cells: the fertility 'bottleneck'
The mid- to late- thirties is still early in life: why is it that women, who are at peak physical health in almost every other regard, have difficulty falling pregnant from this early age? Amazingly, the bottleneck for fertility comes down to a single cell: the egg cell, or "oocyte." While nearly every other tissue in the body is composed of cells that are regularly replaced with fresh cells on a constant basis, humans don't have the ability to generate new oocytes following birth, and women are born with all the oocytes they will ever have. By the time women are trying to fall pregnant, their oocytes are already decades old, and showing the effects of age.
While the ovary eventually does "run out" of oocytes, the decline in oocyte quality occurs much earlier, and is the most important factor in reproduction: while there is a steady decline in pregnancy rates in women undergoing IVF using their own eggs, the success rates of patients who choose to use eggs donated from younger women remain fairly constant, even into later reproductive ages. While there are well-publicised cases of women in their late 40s achieving pregnancy, it is an important public health message that these cases are not common, and are often the result of an oocyte donated from a younger woman. And while using donor oocytes can overcome age-related barriers to reproduction, it comes with ethical issues related to obtaining a donor egg cell, and results in offspring of a different genetic make-up. Not only do these older oocytes result in lower live birth rates, they also result in a higher chance of children born with chromosome disorders.
One strategy that has been used to get around the role of oocyte aging as a limiting factor in female fertility is the use of "egg-freezing," where oocytes are collected from women at a younger age and then frozen, so that when women are ready at a later age to have children, they have access to their own oocytes from a younger age. This service has even been offered as an employee benefit in some Silicon Valley tech companies, with the marketing of this procedure strongly driven by commercial interests. The success of this approach is far from guaranteed, resulting in heart-breaking stories.
It isn't just egg cells that decline with age: male aging is also to blame, with an increased risk of sperm defects that prevent sperm from getting to and into the oocyte. But there is a clear clinical solution to this: intracytoplasmic sperm injection (ICSI), where a very fine needle is used to inject a single sperm cell directly into the egg cell, which occurs under a microscope in a lab. So while male factor infertility is still an important issue, for the most part it is still oocyte quality that is hardest to get around.
Can we improve egg cell quality?
While hormone treatment can increase the number of oocytes released from women, there are no treatments that improve oocyte quality: this is a key goal of the field of reproductive aging.
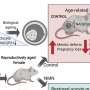
Recently my team—including Ph.D. students and colleagues at UQ and Harvard—teamed up with researchers in reproductive medicine to identify a new cause of poor oocyte quality with age, published today in the journal Cell Reports. We identified declining levels of a key molecule that is essential to metabolism and genome stability in oocytes. When these levels were restored by simply adding the compound back into the drinking water of reproductively aged, infertile lab mice, this restored oocyte quality, allowing these older animals to have babies again. While this is still only an animal study, it is an important proof of concept showing that it might be possible to reverse aspects of female infertility, rather than simply slow the decline of reproductive aging.
It's also important to note that in the absence of clinical evidence, we caution against the use of supplements marketed at women who are desperately trying to get pregnant. Surprisingly, the use of medications, supplements and expensive technical or surgical interventions (collectively termed "IVF add-ons") that do not have strong clinical evidence for improving pregnancy outcomes is widespread in reproductive medicine, driven by patient desperation and the absence of any alternatives. Obtaining solid clinical evidence for these medicines or supplements in patients is a key goal of the Fertility Research Centre, a clinic that was recently opened by the UNSW School of Women and Children's Health and the Royal Hospital for Women in Randwick.
In the long term, we hope that our new strategy could one day be used to improve pregnancy success rates in couples who face the devastating prospect of infertility, reducing the need for repeated rounds of invasive IVF cycles that can lead to emotional, relationship and financial stress. The expensive human clinical trials needed to translate these findings will be a long road ahead—and until they have been completed, we still don't know whether our strategy will work, or even be safe in humans. In the meantime, we and others in the field are conducting basic research in the molecular biology of reproductive aging, with the hope of improving outcomes for mothers and their babies.
More information: NAD+ repletion restores female fertility during reproductive ageing, Cell Reports. DOI: 10.1016/jcelrep.2020.01.058