Math models add up to improved cancer immunotherapy
A merger of math and medicine may help to improve the efficacy of immunotherapies, potentially life-saving treatments that enhance the ability of the patient's own immune system to attack cancerous tumors.
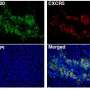
By creating mathematical models that represent the complex interactions within the tumor microenvironment (TME) - the non-mutated cells, connective tissues and blood vessels inside a malignant tumor—researchers at Massachusetts General Hospital (MGH) and Harvard Medical School (HMS) can predict how tumors may respond to immunotherapy, and how adding other anti-cancer drugs could lead to improved treatment. In addition, the models suggest that the relative health of a tumor's blood supply could predict how that tumor will respond to immunotherapy.
Their work is described online in Proceedings of the National Academy of Sciences.
Immune checkpoint inhibitors such as Keytruda (pembrolizumab) and Opdivo (nivolumab) have greatly improved treatment for more than a dozen malignancies, including non-small cell lung cancer, kidney cancer and melanoma, but even in these cancers only a minority of patients benefit from these immunotherapies.
"An estimated 87% of patients currently do not derive long-term benefit from immune checkpoint blocker monotherapy. Therefore, new therapeutic strategies are needed to improve the response rates in patients who are resistant to immune checkpoint inhibition," explains co-author Rakesh K. Jain, Ph.D., from the Edwin L. Steele Laboratories in the Department of Radiation Oncology at MGH and HMS.
Impaired blood perfusion (the flow of blood through vessels in the tissues) is a common feature of many tumor types that limits the ability of drugs to reach malignant cells and results in hypoxia—abnormally low oxygen levels that can in turn lead to suppression of the immune response. To address this problem, Jain and colleagues used a combination of computational and systems biology techniques to develop a model to determine whether "normalization" of the blood vessels and stroma (connective tissues) in the TME could improve the efficacy of immunotherapy.
Although other researchers have developed systems-level mathematical models to predict tumor response to immune checkpoint inhibitors, theirs is the first to incorporate crucial components and interactions of cells with the TME as well as known mechanisms of immune response to explain how the TME might adversely affect the efficacy of immunotherapy, and to predict tumor response to checkpoint inhibitors.
Importantly, the study also points to potential strategies for normalizing the TME to improve the response to immunotherapy. For example, normalization of the stroma with common drugs for treating high blood pressure could improve treatment of desmoplastic tumors, which are marked by dense tissues and compressed, highly abundant but disorganized blood vessels. Conversely, perfusion in tumors with open, leaky blood vessels could be improved with low-dose anti-angiogenic drugs currently on the market, allowing for better delivery of immunotherapy to target tissues.
Triantafyllos Stylianopoulos, Ph.D., co-corresponding author from the University of Cyprus, adds "the identification of tumor perfusion as key to the efficacy of immunotherapy suggests that perfusion could serve as a biomarker of response to immunotherapeutic agents."
More information: Fotios Mpekris el al., "Combining microenvironment normalization strategies to improve cancer immunotherapy," PNAS (2020). www.pnas.org/cgi/doi/10.1073/pnas.1919764117