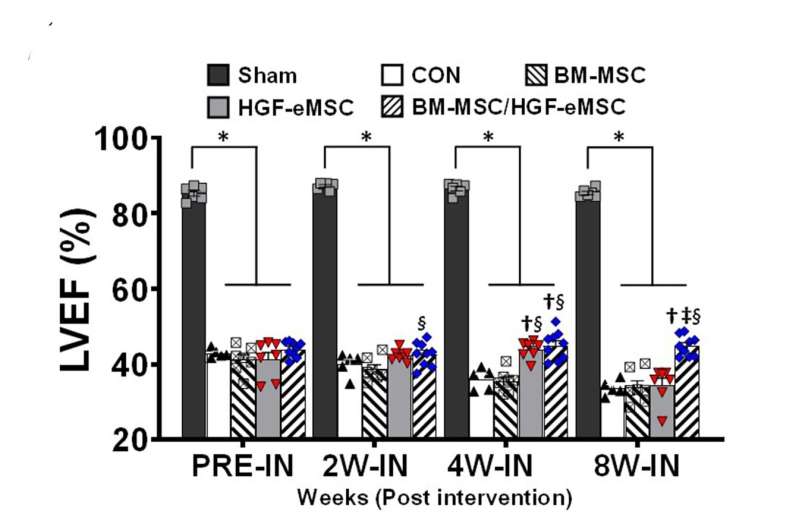
This graph shows the comparison of heart functions in different experimental groups: (i) healthy heart (Sham) ; (ii) untreated myocardial infarction control (CON); (iii) human bone marrow derived-MSCs (BM-MSC) only, (iv) genetically engineered MSCs only (HGF-eMSC), and (v) BM-MSC+ HGF-eMSC mixture. The mixture group shows the best result in left ventricular ejection fraction (LVEF) among the groups using MSCs, which means an enhancement in cardiac function. For example, after 8 weeks, the mixture group shows about 50% of left ventricular ejection fraction, while the untreated control group only shows nearly 32%. Credit: Science Advances; 10.1126/sciadv.aay6994
Human stem cells have been regarded as a promising cell source for cardiac regeneration therapy. But their clinical use is hampered due to poor performance after transplantation into failing hearts. Recently, a stem cell biologist from City University of Hong Kong (CityU), together with his collaborators, has developed a novel strategy called in vivo priming to "train" the stem cells to remain strong after implantation to the damaged heart via a 3-D-printed bandage-like patch. The positive results of the study show that an in vivo priming strategy can be an effective means to enhance cardiac repair.
Dr. Ban Kiwon, Assistant Professor of CityU's Department of Biomedical Sciences, collaborated with cardiologist and experts in 3-D printing from South Korea in achieving this breakthrough. The study was published in the latest issue of Science Advances, titled "In vivo priming of human mesenchymal stem cells with hepatocyte growth factor-engineered mesenchymal stem cells promotes therapeutic potential for cardiac repair."
Harsh environment in failing hearts hinders stem cell survival
Regeneration is a promising strategy to treat myocardial infarction by injecting human stem cells directly into the failing hearts. In particular, human mesenchymal stem cells (hMSCs) have been considered as a competitive agent for clinical uses for their proven safety and significant paracrine effects supporting new blood vessel formation and inhibiting cell death. However, "the clinical trial results are disappointing as the micro-environment of a failing heart is very harsh for the injected hMSCs to stay alive," said Dr. Ban.
Therefore, researchers have been exploring ways to increase the survival rate of hMSCs in failing hearts. "Priming, or so-called preconditioning, is a common strategy to empower the cells. The cells are educated through particular stimulations, and when they are relocated to tough environments, they are much stronger against bad conditions and know how to react because of their previous experiences," explained Dr. Ban.
Conventionally, priming is performed in vitro (outside a living organism) before the cells are transplanted into the heart. "But the effects of priming done in this way usually last for two or three days only. To extend the duration of the priming effect, I have come up with an idea of in vivo priming, which means the hMSCs are primed directly on the failing hearts," said Dr. Ban.
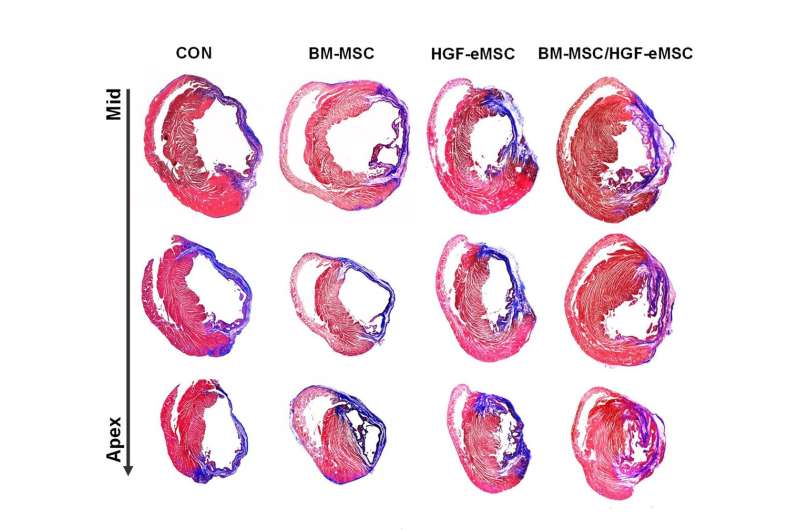
For the group mixing bone marrow-derived MSCs and HGF-genetically engineered MSCs (right column), cardiac tissue harvested at 8 weeks after implanting the patch exhibits substantially smaller areas of fibrosis (scar formation, the parts in blue) than the other experimental groups. That means the patch helps in maintaining the heart's function. Credit: Science Advances; 10.1126/sciadv.aay6994
Novel strategy: in vivo priming of hMSCs
To prove the concept, the research team made a 3-D-printed patch loaded with human bone marrow-derived MSCs, and genetically engineered MSCs with human hepatocyte growth factor protein. Hepatocyte growth factor (HGF) is involved in multiple biological activities, such as cell survival, blood vessel formation and anti-fibrotic activities, and are important in adult organ regeneration and wound healing.
The patch was then implanted like a bandage on the top of the infarct area of the myocardial-infarction-induced hearts of rats. "The genetically engineered MSCs can continuously secret human HGF protein to prime the hMSCs within the patch and make them stronger," said Dr. Ban.
Instead of directly injecting the genetically engineered cells into the heart, he added that encapsulating the cells in the patch for placement on the surface of the heart can prevent mutations or other undesirable outcomes. And the patch is fabricated by 3-D-printing of pig heart-derived extracellular matrix hydrogel, simulating the cardiac tissue-specific micro-environment.
It was found that the primed hMSCs had a higher survival rate compared with unprimed ones in the patches attached to the failing hearts. Those empowered hMSCs released greater amounts of paracrine factors beneficial for repairing damaged cardiac muscle tissues and regenerating vasculatures.
"We found that the primed cells can survive even after eight weeks in the patch after implantation to the heart. Also, there is a significant improvement in cardiac function as well as vessel regeneration comparing to the unprimed cells," said Dr. Ban.
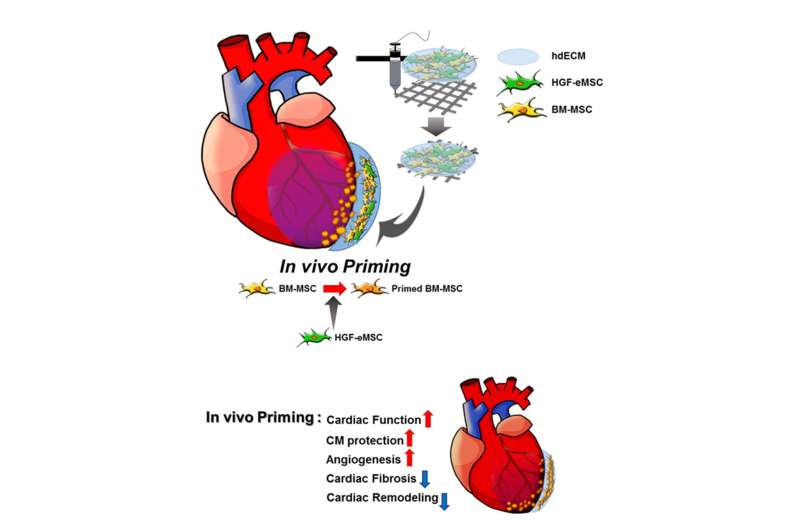
Schematic diagram of the underlying mechanism of in vivo priming. The cardiac patch is 3D-printed with a bioink produced by the mixture of porcine heart-derived extracellular matrix and the MSCs. The patch is then implanted on the infarct area of the rat heart. Credit: © City University of Hong Kong / Science Advances
"Our team is the very first to achieve priming in hearts in vivo. But more importantly, by showing that in vivo priming of hMSCs can enhance the therapeutic potential for cardiac repair, we hope our study can bring significant implications for related stem cell therapy in future," concluded Dr. Ban. It took the team over two years to achieve these remarkable results. The team will explore the possibility of conducting the experiments on bigger animals and even clinical trials, as well as modifying the structure of the patch.
More information: "In vivo priming of human mesenchymal stem cells with hepatocyte growth factor–engineered mesenchymal stem cells promotes therapeutic potential for cardiac repair" Science Advances (2020). DOI: 10.1126/sciadv.aay6994 , advances.sciencemag.org/content/6/13/eaay6994
Journal information: Science Advances
Provided by City University of Hong Kong