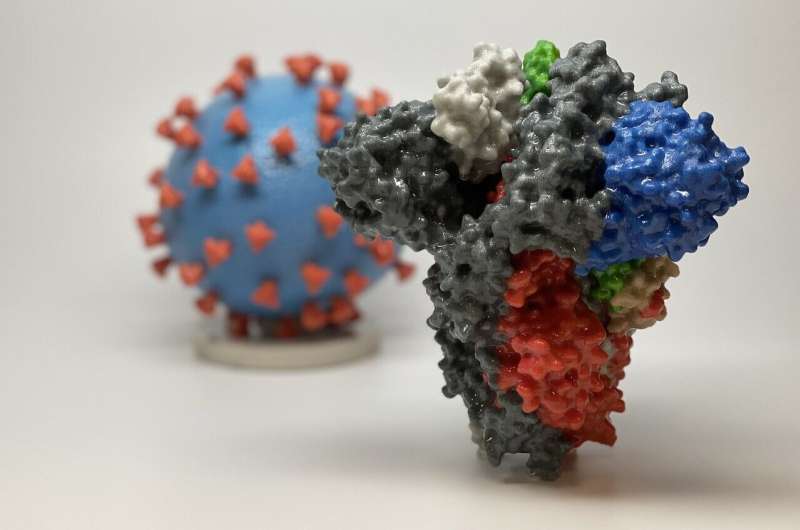
3D print of a spike protein of SARS-CoV-2, the virus that causes COVID-19—in front of a 3D print of a SARS-CoV-2 virus particle. The spike protein (foreground) enables the virus to enter and infect human cells. On the virus model, the virus surface (blue) is covered with spike proteins (red) that enable the virus to enter and infect human cells. Credit: NIH
As the new coronavirus continues to claim lives, the race is on to develop fast, convenient and accurate diagnostic tests for COVID-19. Now, researchers from CAS, a division of the American Chemical Society specializing in scientific information solutions, have compiled a special report published in ACS Central Science. Drawing from published journal articles and a variety of other published resources, this report provides a detailed overview of COVID-19 diagnostic tests, trends and resources.
According to the World Health Organization, as of April 26, 2020, the COVID-19 pandemic has caused more than 2.8 million confirmed illnesses and more than 193,000 deaths. Social distancing requirements and business lockdowns have slowed the virus' spread, but at the same time, these measures have disrupted people's lives and weakened economies. To help prevent future outbreaks of COVID-19, experts agree that fast, convenient and accurate diagnostic tests are desperately needed. Widespread testing of the general population would allow public health officials to identify and isolate patients early in the course of their illness, as well as asymptomatic people who might unknowingly spread the disease. To help better understand the numerous diagnostic tests available, a group of CAS scientists led by Cynthia Liu summarized the basic principles of molecular and serological assays underlying these tests. The researchers also provided a high-level view of the more than 200 diagnostic tests currently available.
Tests for COVID-19 currently fall into two major categories: those that detect the RNA of SARS-CoV-2, the virus that causes COVID-19; and those that detect antibodies in the blood of people who at some point were infected with the virus. In the first category, the most common tests rely on the reverse-transcriptase-polymerase chain reaction (RT-PCR), which amplifies a tiny amount of viral RNA collected from nasopharyngeal swabs. Because RT-PCR requires expensive instruments, trained personnel and often several days to generate results, researchers are avidly exploring other methods, such as isothermal nucleic acid amplification and transcription-mediated amplification, as well as CRISPR technologies. The second category of tests cannot be used for early diagnosis because antibodies do not appear in the blood for days to weeks after infection. However, serological and immunological assays could be used to confirm suspected cases, monitor the progress of an individual's disease, or identify people with past infection and potential immunity. Scientists are researching many different types of assays, such as the traditional enzyme-linked immunosorbent assay (known as ELISA), lateral flow immunoassays and surface plasmon resonance-based biosensors. Widespread deployment of both categories of tests could help manage people's return to normal activities, but many questions, including the specificity and sensitivity of the tests, remain to be answered, the researchers say.
More information: Linda J. Carter et al. Assay Techniques and Test Development for COVID-19 Diagnosis, ACS Central Science (2020). DOI: 10.1021/acscentsci.0c00501
Journal information: ACS Central Science
Provided by American Chemical Society