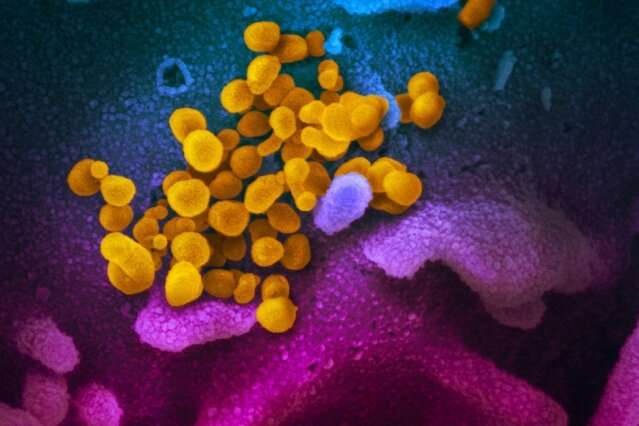
This scanning electron microscope image shows SARS-CoV-2 (yellow)—also known as 2019-nCoV, the virus that causes COVID-19—isolated from a patient, emerging from the surface of cells (blue/pink) cultured in the lab. Credit: NIAID-RML
Researchers across the world have responded rapidly to COVID-19—a new disease that we don't yet know how to treat. In the search to find safe and effective treatments, teams of scientists are looking into more than 200 potential options(opens in a new tab), running thousands of studies(opens in a new tab) around the world. But they need support and ways to coordinate their work. That way, they will stand the best possible chance of getting reliable results sooner.
What are the potential treatments?
The coronavirus that causes COVID-19 is a completely new virus, which we still know too little about. So researchers are taking a wide a range of approaches to find an effective and safe treatment.
The three main approaches are: antivirals, anti-inflammatory drugs and antibody treatments.
Antivirals
Antiviral drugs work by preventing a virus from developing inside the human body.
Every virus is different and attacks cells in specific ways—and the antiviral drugs that fight them off are specific too. Very rarely does an antiviral built for one virus also work for different ones. But it can happen, for example, some HIV drugs have also proved effective in fighting off hepatitis B.
It would be great to have an antiviral specific to COVID-19, but that could take years to discover. In the meantime, researchers are hopeful that some existing antivirals, whether already on the market or experimental, could have some useful effect against the novel coronavirus. These include:
- Remdesivir, an antiviral tested as an Ebola treatment. It has generated promising results in animal studies for Middle East Respiratory Syndrome (MERS-CoV) and severe acute respiratory syndrome (SARS), which are also caused by coronaviruses, suggesting it may be some use against COVID-19.
- Lopinavir-Ritonavir, a combination of antivirals used to treat HIV. Laboratory experiments have suggested some effect against COVID-19, but studies in humans have so far been inconclusive.
- Chloroquine and hydroxychloroquine, drugs used to treat malaria and rheumatology conditions. Small studies have given some indications of possible antiviral benefit against COVID-19, but the World Health Organization has recently warned about safety concerns. The evidence is far from clear, and large scale clinical trials are needed to give a definitive answer.
How COVID-19 treatments could work
Anti-inflammatories
Anti-inflammatory drugs work by calming the immune system. In people with severe COVID-19, the body's violent reaction in trying to fight off the virus can cause serious harm and even death. Anti-inflammatories can reduce this response. Some of the possibilities researchers are looking into are:
Interferon beta-1a, which is used to treat multiple sclerosis by stopping the immune system from damaging the coatings of nerve cells. Interferons have previously been found to show some effects against MERS-CoV and SARS, which are also caused by coronaviruses.
Dexamethasone, which is a type of steroid used to reduce inflammation in a range of conditions, including sore throats.
Antibody treatments
Antibodies attack the virus directly. Unlike antivirals and anti-inflammatory drugs, antibodies are produced naturally by people who have had an infection and recovered. When given to patients who are fighting off an infection, antibodies can boost their immune response and stop the virus from causing further harm.
There are two ways that antibodies can be used:
Convalescent plasma can be extracted from the blood of COVID-19 survivors and injected into patients who are fighting the disease. This may help, but the only source of it is blood donors who have had the infection, so there will not be nearly enough supply to treat a large number of patients. Hospitals around the world are already using this approach, but these efforts are necessarily local and small-scale.
Monoclonal antibodies are antibodies specific to COVID-19. While they also originate in the blood of people who have recovered, that is only the starting-point. Scientists select the relevant antibodies, extract and expand them, and then manufacture them in large quantities for widespread use. No clinical studies have started yet, but early-stage work is well underway in numerous labs worldwide.
How do we find out whether these treatments work?
- Potential new drugs have to go through years of laboratory work, animal testing and human clinical trials before they're proved to be safe and effective against a specific disease. So developing a new antiviral against the novel coronavirus is not the best hope for containing the ongoing pandemic.
- This is why researchers have been looking into existing drugs, some experimental and some already licensed, that were developed for other infections, such as Ebola and malaria. These drugs have a head start and we can establish more quickly whether they are useful against COVID-19.
- Experimental drugs, such as remdesivir, may have already been tested in animals for safety. But they usually have to go through safety trials in humans, to assess what the safe dose is, before moving into clinical trials to assess how effective they are.
- Existing antivirals have already been proved to be safe for humans, at a specific dose—so what needs to be done now is test whether they work in people with COVID-19. Although there are many small studies investigating the effectiveness of drugs like chloroquine, these can only give indications, not results that are conclusive enough to justify their approval. What we need are large randomised controlled trials, with hundreds or thousands of people enrolled across the world, to tell us whether existing antivirals bring benefits to people with COVID-19.
- Existing anti-inflammatory drugs, such as interferon beta-1a, are also already known to be safe if used in certain ways. But because of the way these drugs interact with the immune system, we need more data to understand whether they are safe in the context of a COVID-19 infection. This can be done through small studies first, to assess what the right dose is, before moving into large randomised controlled studies to test their effectiveness.
- Monoclonal antibodies are a new potential treatment, so they also have to go through safety trials in humans. But because these are natural antibodies originating in human bodies, not synthetic compounds, safety trials will take less time to conduct. After that, effectiveness studies can get going.
Large randomised clinical trials—such as the World Health Organization's Solidarity clinical trial(opens in a new tab) and the UK's Recovery trial(opens in a new tab) – are essential for getting robust data about which treatments work against COVID-19. Both of these are looking at several treatments, including some of those listed above, but the studies are designed to be adaptable ongoing platforms—so they can quickly stop testing drugs that prove not to be working, and add new ones as they develop.
How do we make treatments accessible to all?
COVID-19 has reached every corner of the world, and it will keep spreading unless it is controlled everywhere. To stop the pandemic, to get societies back to normal, and to get economies moving again, any treatments and vaccines must be made accessible to everyone who needs them, everywhere in the world, regardless of their ability to pay.
For that to happen:
- governments, industry and philanthropy must pool resources to pay for the risk, the research, manufacturing and distribution to ensure everyone has access to treatments
- clinical trials need to take place across the world, to make sure treatments work for everyone
- national governments must work together to ensure that coronavirus treatments can be manufactured in as many countries as possible and distributed globally to everyone who needs them.
Provided by Wellcome Trust