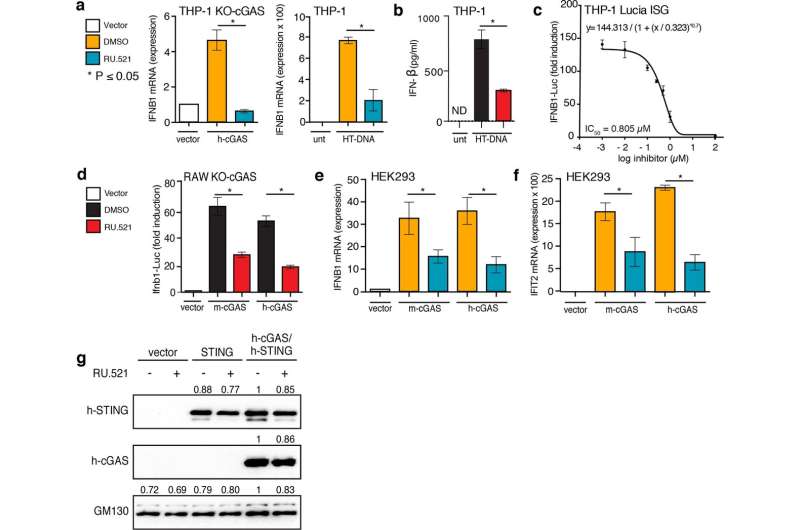
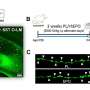
Inhibition of human and mouse cGAS with RU.521 reduces interferon expression in multiple cell types. Lines used were: THP-1 and its derivatives (a–c), RAW 267.4 (d), and HEK293 (e–g) cells. THP-1 KO-cGAS cells were transfected with either vector plasmid control or a plasmid containing h-cGAS, and treated with either vehicle (DMSO) or RU.521 (a, left panel). WT THP-1 cells were mock treated (unt) or stimulated with 0.3 µg/mL HT-DNA +/− RU.521, followed by RT-qPCR analysis for IFNB1 expression (a, right panel). An ELISA for human IFNB1 was also performed on THP-1 cells stimulated with HT-DNA +/− RU.521 (0.8 µM) (b). A dose-response curve was generated for RU.521 using immune-activated THP-1 Lucia ISG cells (c). RAW-Lucia ISG KO cGAS cells were transfected with plasmids expressing WT m-cGAS or h-cGAS, and activity was read via luciferase reporter (d). HEK293 cells were transfected with human or mouse cGAS in addition to h-STING, in the presence or absence of RU.521. The RNA levels for IFNB1 and IFIT2 were then measured by RT-qPCR (e,f). Transfection of vector backbone was used to normalize the data. Immunoblot analysis of transfected h-STING, h-cGAS and m-cGAS into HEK293 cells +/− RU.521 shows protein expression is unaltered by addition of the small molecule. GM130 was used to normalize the data (g). The minimum numbers of biological and technical replicates for the assays were three and three, respectively (n = 9). Error bars represent SEM. Asterisks (*) denote P ≤ 0.05 where an unpaired t-test with Welch’s correction was used to compare the results. Credit: Scientific Reports (2020). DOI: 10.1038/s41598-020-64348-y
The protein cGAS plays an essential role in cellular innate immunity by detecting the DNA of invading pathogens such as bacteria and viruses, or our own damaged and mislocalized DNA. Activation of the cGAS-STING signaling pathway produces a pro-inflammatory immune response, and prolonged activation of cGAS can result in lupus-like autoimmune disorders.
Manuel Ascano, Ph.D., and colleagues previously identified the compound RU.521 as an inhibitor of mouse cGAS. They now demonstrate that RU.521 also potently and selectively inhibits human cGAS in cell lines and in human primary cells. They showed that RU.521 suppressed cGAS activity in a dose-dependent manner and did not suppress immune responses activated by non-DNA signals.
The study, published May 5 in Scientific Reports, validates the use of RU.521 as a tool to assess the contribution of the cGAS-STING signaling axis in various immune responses. The compound could also serve as a chemical scaffold for the development of potential therapeutics for cGAS-related autoimmune diseases.
More information: Caroline Wiser et al. Small molecule inhibition of human cGAS reduces total cGAMP output and cytokine expression in cells, Scientific Reports (2020). DOI: 10.1038/s41598-020-64348-y
Journal information: Scientific Reports
Provided by Vanderbilt University