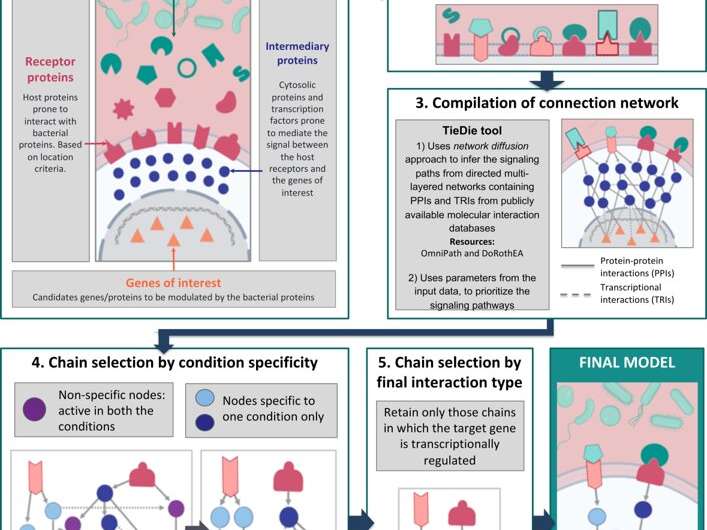
The MicrobioLink workflow. Credit: Cells 2020, 9(5), 1278; https://doi.org/10.3390/cells9051278
A new tool developed by scientists at the Quadram Institute and Earlham Institute is helping to translate the complex communication between the microbiome and the body. The tool will be valuable for researchers trying to understand microbes influence health, the changes that lead to disease, and point to potential drug targets.
The human gut microbiome has important roles in keeping us healthy. It's vital for obtaining nutrients from food, and it's becoming more apparent it plays a role in different chronic, terminal and lifestyle diseases. Changes in the composition of the microbiome have been associated with type 2 diabetes, obesity and inflammatory bowel disease (IBD), but it's not clear how those changes cause the onset of different conditions, or whether they are instead a symptom.
To start unpicking the mechanisms, researchers have been focusing on the communication between microbes and their host organisms, including humans. Microbes release a variety of metabolites, proteins and RNA molecules that allow them to communicate with other proteins from the host organism. This may elicit a response from the host networks resulting in turning on or off host genes that modulate various processes.
Translating these cross-kingdom communications between microbial and host proteins is vital if we are to understand the processes that affect health as well as disease. Such inter-kingdom communication studies have been done for some bacteria, mainly pathogenic species that trigger specific disease conditions. But the microbiome may contain thousands of different species, all potentially communicating with an array of different host proteins, linked to many different signalling pathways and physiological processes.
To get a handle on these complex communications, Dr. Tamas Korcsmaros and Dr. Padhmanand Sudhakar from the Quadram Institute and the Earlham Institute and colleagues developed an integrated computational pipeline called MicrobioLink. Published in the journal Cells, MicrobioLink connects microbial proteins with host proteins they are likely to interact with, and then infers how these interactions influence cellular processes in the host.
Researchers can use Microbiolink to test a set of proteins, which for example might be the proteins secreted by an entire microbial community, against a relevant list of host proteins from experimental data or existing databases. The pipeline then predicts interactions between microbial and host proteins based on their respective molecular features such as domains and motifs. Protein-protein interactions represent potentially interesting communication channels between bacteria and host.
The power of Microbiolink comes in the next step that uses a principle,termed diffusion, in network science to trace the effects of the interactions between the microbial and host proteins on other host processes further downstream. This uses existing databases of validated molecular interactions to follow signals from host proteins (predicted to be bound by and hence modified by microbial proteins) through to other target genes or proteins in the host. With appropriate filtering, this can identify key nodes in networks and specific genes or proteins in the host that are affected by the original communication between the microbes and the host.
To test the pipeline, the research team carried out a case study using a compendium of bacterial proteins found only in patients with Crohn's disease, and another set of proteins found only in healthy people. They used MicrobioLink to compare how the different sets of proteins affected autophagy, which is an important cellular process that's known to be dysregulated in Crohn's disease. This revealed a network with clearly separated signalling paths exclusive to the disease and healthy contexts and, ultimately, influencing the expression of autophagy genes.
These findings would need to be confirmed experimentally but they point towards a potential mechanism linking the microbiome and Crohn's disease. The case study also demonstrates the potential for this integrated computational pipeline to translate communications between microbes and host so we can start to understand their effects on the body, and their implications on health and disease.
As well as studying the influence of the microbiome on other diseases, MicrobioLink could also be used to unpick the beneficial effects on the host of probiotic bacteria or communities of bacteria, and not just in humans.
More immediately, the team is looking to apply MicrobioLink to understanding the effects of the SARS-CoV-2 virus behind the COVID-19 pandemic.
More information: Tahila Andrighetti et al. MicrobioLink: An Integrated Computational Pipeline to Infer Functional Effects of Microbiome–Host Interactions, Cells (2020). DOI: 10.3390/cells9051278
Provided by Quadram Institute