Elderly, sick, essential workers will get virus vaccine first: US
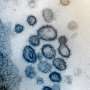
The United States plans to give priority to the elderly, those with pre-existing conditions and essential workers once there is a vaccine against COVID-19, a senior Trump administration official said Tuesday.
Under Operation Warp Speed (OWS), the US government is aiming to deliver 300 million vaccine doses by January 2021, investing in manufacturing capacity to relieve pharmaceutical firms of the financial risk.
"Before any vaccines are approved or authorized, Operation Warp Speed will build the necessary plans and infrastructure for distributing them," the official said.
Officials envision distributing COVID-19 in tiers, employing a decade-old methodology used for pandemic influenza.
"The elderly, those with pre-existing conditions and people performing essential services would be given higher tiers," the senior official said.
"However, which populations are able and should receive a vaccine that is developed will depend on the results of clinical trials," the official added.
A second official stressed that the safety of vaccine candidates is not yet known, and it may turn out that they are not suitable for certain demographics.
In addition, that official said, "we fully expect there will be—name the number—20, 30, 40 million Americans that probably have strong antibodies to coronavirus by the end of the year, so they would be a significantly lower priority."
While the development of a strong vaccine is not 100 percent guaranteed, the officials said the goal is to have enough vaccines by the height of next year's flu season to vaccinated those who are vulnerable and desire a vaccine.
Insurance companies have said they intend to make the vaccines available to clients without an additional charge, the officials added.
Operation Warp Speed, which was announced May 15, also is aimed at accelerating the development and deployment of COVID-19 treatments and diagnostics.
Some of the leading vaccine candidates include one developed by AstraZeneca in conjunction with the University of Oxford, which will enter the final stages of its testing this summer.
The Health and Human Services (HHS) department has invested $1.2 billion in the vaccine and reached an agreement to make 300 million doses available to the United States, the first of which could be delivered as early as October 2020.
HHS has likewise invested $456 million in Johnson & Johnson's vaccine, set to begin human trials this summer, and $483 million in Moderna's vaccine, which will enter its final trial phase in July.
© 2020 AFP