July 7, 2020 feature
Four-dimensional physiologically adaptive cardiac patch
Bioengineers have considerably advanced cardiac scaffold engineering techniques to treat myocardial infarction, a form of cardiovascular disease and the leading cause of morbidity and mortality worldwide. However, it is still challenging to replicate structural specificity and variability of cardiac tissues with traditional bioengineering processes. In a new report on Science Advances, Haitao Cui and an interdisciplinary research team at the National Institute of Health, University of Maryland, and the George Washington University, U.S. developed a four-dimensional (4-D) cardiac patch with physiological adaptability. They used beam scanning stereolithography to print the construct and combined 4-D self-morphing with expandable microstructures to improve their biomechanical properties to integrate within the beating heart. The results showed improved vascularization and maturation of cardiomyocytes under physiologically relevant mechanical stimulation. The constructs were suited for use in a mouse model of chronic myocardial infarction (MI) with improved cell engraftment and vascular supply. The work provides an effective treatment strategy for MI and a cutting-edge bioengineering method to improve the structural design of complex tissues for organ regeneration.
Bioengineering the human heart
The heart is a dynamic and multicellular tissue-bound organ with highly specific structural and functional characteristics. Adult cardiac muscles lack the ability to self-repair and regenerate after MI, therefore traditional cardiac patches serve as temporary mechanical supporting systems to prevent post-infarction left ventricular remodeling. The field of cardiac engineering is emerging to generate functional cardiac tissues as a long-term, promising alternative to repair damaged tissue. The scaffold can provide mechanical support with cellularized patches and restore functionality of the damaged myocardium. Hydrogel-based materials derived from natural sources are well suited to mimic the tissue microenvironment and support cell adhesion and growth. They provide favorable matrices for growth and differentiation of cardiomyocytes, but their structural design and manufacturing limits have made clinical applications challenging. Most research teams incorporate human induced-pluripotent-stem-cell-derived cardiomyocytes (hiPSC-CMs) as a continuous source of cells for cell differentiation (growth and maturation), due to their promising applications in cardiac engineering.
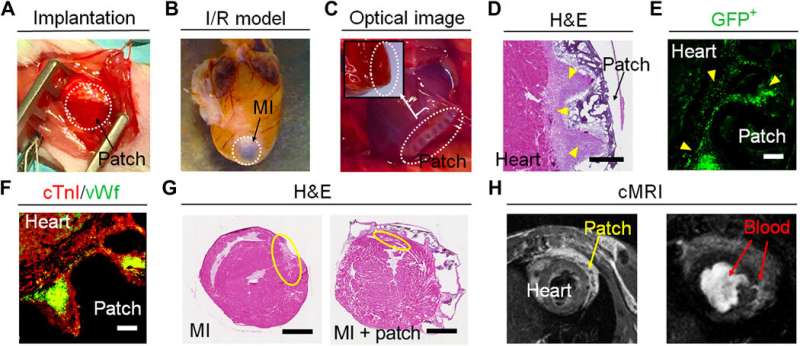
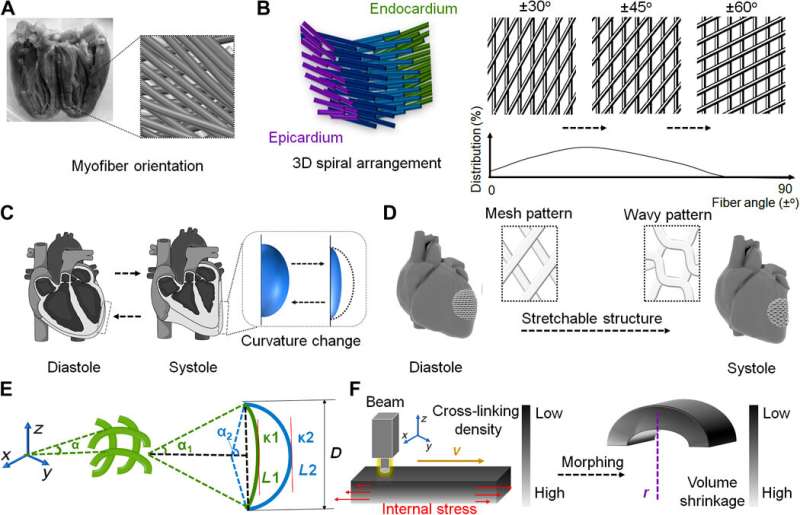
Cui et al. developed a 4-D hydrogel-based cardiac patch with a specific smart design for physiological adaptability using beam scanning stereolithography to achieve many specific micropatterns and microarchitectures. The self-morphing process achieved conformations identical to the surface curvature of the heart. The scientists accounted for the physiological features of the cardiac tissue to create a highly stretchable microarchitecture, using hydrogel for ease of transformation from a wavy to mesh pattern—relative to diastole and systole functions of the cardiac cycle. The team then tri-cultured cardiomyocytes, mesenchymal stromal cells, and endothelial cells on the engineered cardiac patches to reproduce vascular networks to support and guide contracting cells.
Designing a physiologically adaptable cardiac patch
Using diffraction tensor imaging (DTI), the scientists noted a helical network of myofibers in the left ventricle (LV) arranged to form a sheet structure, while computer-aided design (CAD) helped them transform the anatomical details of complex fiber arrangements to an engineered cardiac tissue. The diastole and systole functions are specific to the cardiac tissue and induced by cardiac muscle contractions, to generate the force for blood circulation. The volume change in the heart accounted for the dynamically stretched arrangement of fibers in a selected region. To account for ventricular curvature, the CAD-derived mesh pattern could therefore change to a hexagon or wavy pattern in the 2-D plane. Cardiac muscle fibers contain longitudinally bundled myofibrils with cardiomyocytes and collagen sheaths, surrounded by high density capillaries. This anisotropic muscular architecture can produce coordinated electromechanical activities of the ventricles such as contraction and propagation of the excitation wave.
![Printing of smart cardiac patch and optimization. (A) Curvature change of 4D morphing versus printing speed (means ± SD, n ≥ 6, *P < 0.05). (B) Printing accuracy of the hydrogel patches versus fiber width for different fill density (fd; means ± SD, n ≥ 6, *P < 0.05, **P < 0.01, and ***P < 0.001). (C) Color map of tensile moduli of the patches with varying GelMA and PEGDA concentrations. (D) Optical and 3D surface plot images of the patches. Scale bars, 200 μm. (E) Average elasticity values of the wave-patterned patches in horizontal (x) and vertical (y) directions. Number sign (#) shows the statistical comparison between the horizontal and vertical directions (means ± SD, n ≥ 6, **P < 0.01 and ##P < 0.01). (F) Uniaxial tensile stress-strain curves of 5% GelMA and 15% PEGDA. Immunostaining of cell morphology (F-actin; red), sarcomeric structure (α-actinin; green), gap junction [connexin 43 (Cx43); red], and contractile protein [cardiac troponin I (cTnI); red] on the patches on (G) day 1 and (H) day 7. Scale bars, 20 μm. (I) Beating rate of hiPSC-CMs on the patch and well plate on day 3 and day 7 (means ± SD, n ≥ 6, *P < 0.05; n.s. no significant difference). BPM, beats per minute. Photo credit: Haitao Cui, GWU. Credit: Science Advances, doi: 10.1126/sciadv.abb5067 Four-dimensional physiologically adaptive cardiac patch](https://scx1.b-cdn.net/csz/news/800a/2020/6-fourdimensio.jpg)
In order to integrate the relationship between the stretchable structure and ventricular curvature, the team mathematically characterized the design using a simplified, solid geometric model with a plane curve prototype. Researchers had previously used light-induced 4-D morphing with customized beam scanning stereolithography to develop laser-induced graded internal stress as a major diving force for 4-D dynamic morphing in neural engineering. Based on these principles, the 3-D printed cardiac patch developed by Cui et al. could transform from a flat pattern to the 4-D curved architecture, depending on the selection of appropriate printing parameters.
Printing and optimizing the cardiac patch
The scientists used a printable ink made of gelatin methacrylate (GelMA) and polyethylene glycol diacrylate (PEGDA) to engineer the anisotropic cardiac patch with myocardial fiber orientation. GelMA is a photocurable biomaterial containing many peptide sequences to promote cell attachment and growth. The printing speed affected the photocuring performance and structural accuracy to prompt 4-D self-morphing. The team determined the printing accuracy of fiber arrangement and varied the weight ratio of GelMA and PEGDA for the resulting mechanical moduli of hydrogels to reach the modulus of the native myocardium, while printed patterns represented the microarchitecture of the native myocardial tissue.
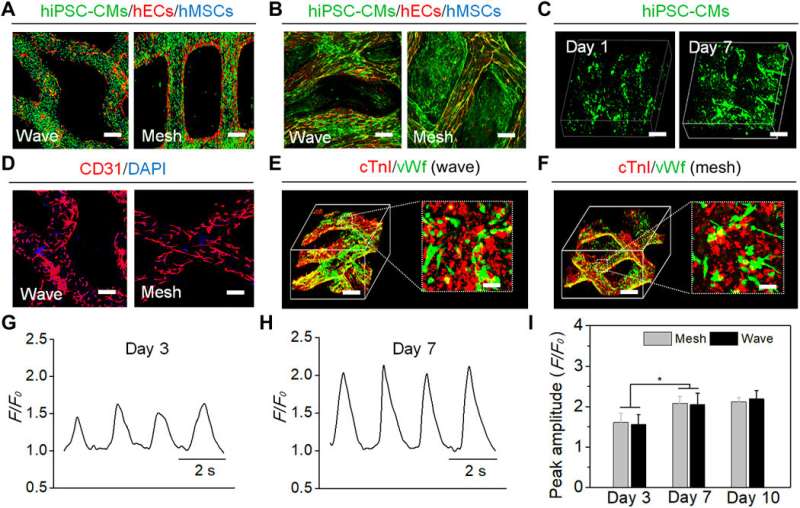
The team cultured hiPSC-CMs on the scaffolds due to their known ability to restore cardiac functions, and after three days, the attached hiPSC-CMs showed spontaneous contractions. Seven days later, the hiPSC-CMs formed aggregates on top of the printed fibers for synchronous contraction for electrophysiological cell coupling. By day seven, the cardiomyocytes showed increased growth on the cardiac patches, while calcium transients increased to a stable state to establish excellent functional contraction-relaxation physiological behaviors.
Functional maturation tests and in vivo implantation
For biomechanical stimulation and functional maturation studies, the team performed immunostaining and determined the expression of cardiac-related genes on cell-material constructs. By day 14, they noted the increased expression of genes associated with excitation-contraction coupling, sarcomeric structure and angiogenesis (formation of new blood vessels), as the iPSC-CMs in the printed patches matured in time. The team implanted the cardiac patches in murine models of ischemic-reperfusion injury for clinically acute or chronic heart disease simulation in cardiovascular research.
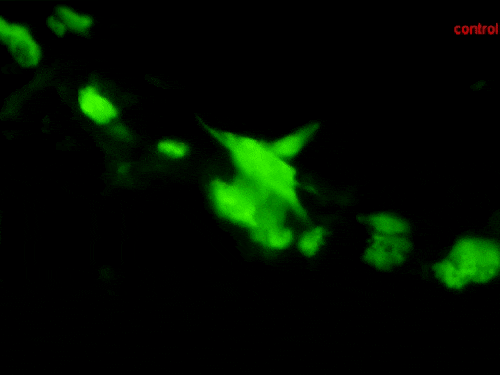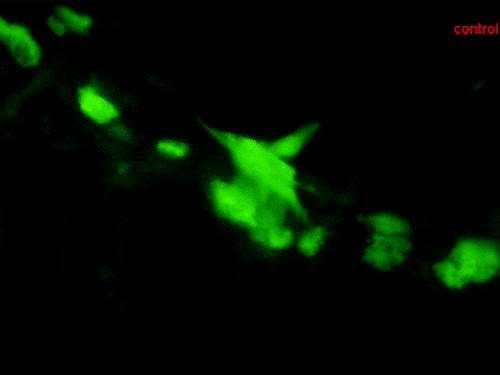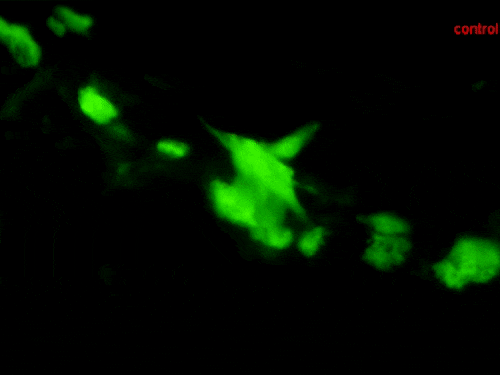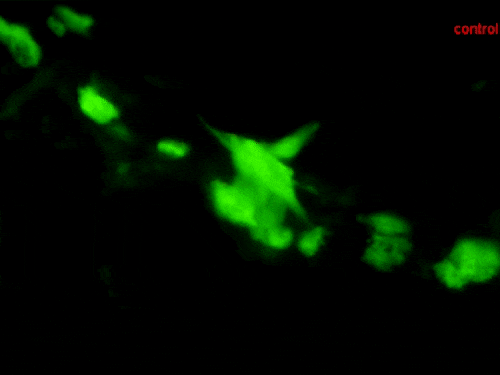
They assessed the cardiac patches in a long-term (four-month) study after implantation to highlight the recovery of animal models within a short period of time with less inflammation and high rates of survival—compared to the classical MI model. The team conducted immunofluorescent assays to show vascular cells spanning the interface of the myocardium and expanded within the myocardial patch, thus providing mechanical support and effectively preventing LV (left ventricular) remodeling. By four months, the implanted patch showed excellent connectivity with the mouse heart, the scientists noted a higher cell density and smaller infract area in the cellularized patch compared to acellular patches implanted in the MI group.
In this way, Haitao Cui and colleagues developed a physiologically adaptable, 4-D cardiac patch to recapitulate the architectural and biological features of the native myocardial tissue. They used beam-scanning SL printing to engineer the smart patches to provide mechanical support and a physiologically tunable structure and matrix for cell implantation. The cardiac patches showed high levels of cell engraftment and vascularization upon implantation in a mouse MI model. The team intend to expand future studies to a physiologically relevant large animal model such as porcine or non-human primate MI model for more realistic results in clinical cardiac engineering therapies.
More information: Haitao Cui et al. 4D physiologically adaptable cardiac patch: A 4-month in vivo study for the treatment of myocardial infarction, Science Advances (2020). DOI: 10.1126/sciadv.abb5067
Michael A. Laflamme et al. Heart regeneration, Nature (2011). DOI: 10.1038/nature10147
Florian Weinberger et al. Engineering Cardiac Muscle Tissue, Circulation Research (2017). DOI: 10.1161/CIRCRESAHA.117.310738
© 2020 Science X Network