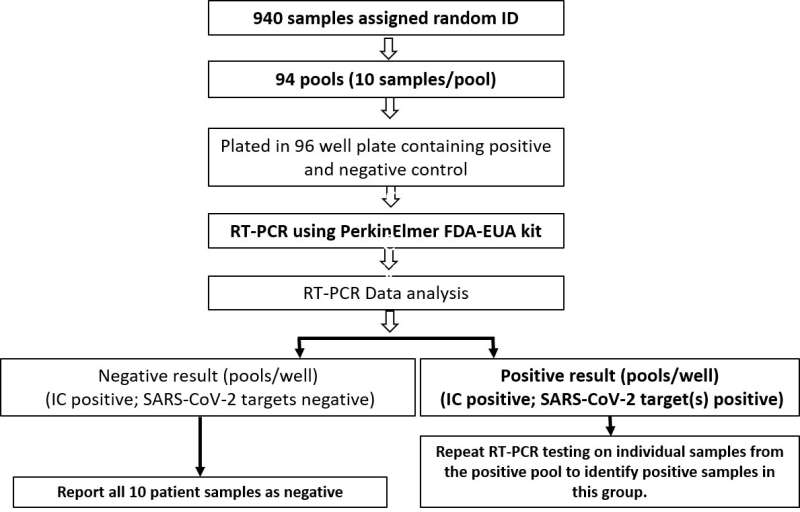
The cost-effective and rapid workflow of the pooling strategy for screening of SARS-CoV-2 in clinical samples for mass population screening. Credit: The Journal of Molecular Diagnostics
In a report in the Journal of Molecular Diagnostics researchers at Augusta University and PerkinElmer Genomics describe a cheaper, rapid, and accurate pooling strategy for the RT-PCR-based detection of SARS-CoV-2 in clinical samples. This assay has a significant impact on large-scale population screening in the wake of the current COVID-19 pandemic.
The COVID-19 pandemic has caused a massive strain on global healthcare systems, governance, and economies. Testing for SARS-CoV-2 has lagged in many countries due to various bottlenecks including but not limited to the failure of the reagent supply chain, lack of adequate test kits, and hindrances in the analytical and regulatory processes.
"We have proposed a mass population screening approach based on sample pooling strategy for rapid and wide-scale population screening that may be adopted by laboratories currently using RT-PCR-based methods to test for SARS-CoV-2," explained Ravindra Kolhe, MD, Ph.D., Vice-Chair for Translational Research in the Department of Pathology, Medical College of Georgia, Augusta University, Augusta, GA, USA.
According to Dr. Kolhe, this strategy would lead to an approximately 5- to 10-fold reduction in the cost of testing of SARS-CoV-2. PerkinElmer's FDA-EUA (Emergency Use Authorization) RT-PCR kit for SARS-CoV-2 detection (with a limit of detection of 5-20 copies/mL) has been implemented in the laboratory by Dr. Kolhe since March 2020.
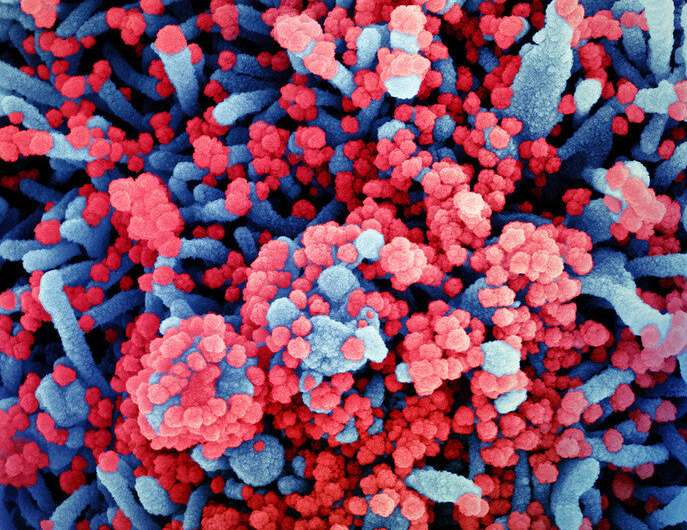
Colorized scanning electron micrograph of a cell (blue) heavily infected with SARS-CoV-2 virus particles (red), isolated from a patient sample. Image captured at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Credit: NIAID
The investigators analyzed approximately 1,000 samples using this pooled sampling approach and achieved 91.6 percent PPA (positive percent agreement) and 100 percent NPA (negative percent agreement) compared to the routine screening approach. The study demonstrates the clinical utility and accuracy of sample pooling strategy and presents a carefully planned layout for mass population testing that can be executed by laboratories using RT-PCR-based methods for detecting SARS-CoV-2. The proposed methodology has the potential to allow institutions to ramp up their testing needs in a cost-efficient manner with minimal turnaround times. This testing allows for the timely isolation and treatment of COVID-19-positive patients, specifically in populations with a lower prevalence of the disease, for example, screening large numbers of students returning to college campuses.
"In terms of cost analysis, one million individuals can be tested for around US$9.1 million with the proposed mass population screening approach compared to approximately US$58 million with current routine diagnostic methods. These cost projections highlight significant savings of healthcare dollars that could be achieved in both high- and low/middle-income countries," said Amyn Rojiani, MD, Ph.D., Chair of the Department of Pathology, Augusta University, Augusta, GA, USA. The global impact of these enormous savings has the potential to enhance laboratory operations throughout the pandemic and can be deployed to laboratories that are facing dire constraints in supplies.
More information: Nikhil Shri Sahajpal et al, Proposal of Reverse Transcription-PCR–Based Mass Population Screening for SARS-CoV-2 (COVID-19), The Journal of Molecular Diagnostics (2020). DOI: 10.1016/j.jmoldx.2020.07.001
Journal information: Journal of Molecular Diagnostics
Provided by Elsevier