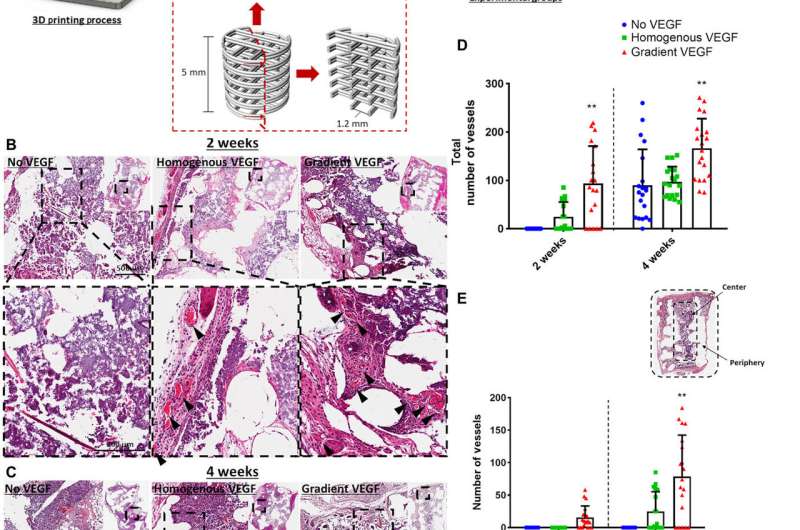
Enhanced vessel infiltration and angiogenesis due to distinct VEGF gradient. (A) Schematic of the 3D printed scaffold and experimental groups. Construct design (4 mm in diameter, 5 mm in height). H&E-stained sections of the three experimental groups at (B) 2 and (C) 4 weeks in vivo. Images were taken at 20×. Arrows denote vessels. (D) Total number of vessels of the experimental groups at 2 and 4 weeks in vivo. Number of vessels present in the center versus the periphery at (E) 2 and (F) 4 weeks in vivo. **P < 0.01. Error bars denote SDs (n = 8 animals, n = 5 slices per animal). FBS, fetal bovine serum; pen/strep, penicillin/streptomycin. Credit: Science Advances, doi:10.1126/sciadv.abb5093
A team of researchers in biomedical engineering, mechanical engineering and biomechanics in Ireland, the Netherlands and the U.S. recently developed 3-D bioprinted implants optimized with growth factors to facilitate angiogenesis—blood vessel growth from existing vasculature—and osteogenesis—new bone growth. In this work, Fiona E. Freeman and colleagues functionalized bio-inks with nanoparticles to print implants with distinct growth factor patterns and noted how the rate of in vivo blood vessel growth (angiogenesis) depended on the expression of the vascular endothelial growth factor (VEGF). They noted higher levels of vessel invasion in implants containing a gradient of VEGF compared to those homogeneously loaded with similar amounts of the protein. The printed implants maintained a gradient of VEGF coupled with spatially defined bone morphogenetic protein (BMP) localization and release kinetics to accelerate large bone defect healing alongside minimal heterotopic bone formation i.e. abnormal bone growth in nonosseous tissue. The work has potential to bioprint growth factors for point of care therapy and in biomedical applications of tightly controlled tissue regeneration.
Growth factors for therapeutic applications
Researchers have tested a number of growth factors in clinical trials for varying therapeutic applications including bone regeneration and new blood vessel formation or neovascularization. Despite promising results, some outcomes in phase two-trials have not shown expected benefits to patients and some have even demonstrated marked adverse effects. Growth factors are typically expressed during different phases of fracture healing, including vascular endothelial growth factor (VEGF) and bone morphogenetic protein (BMP). For optimal outcomes scientists have developed combination therapies of the two growth factors (VEGF and BMP) to accelerate the regeneration of large bone defects. During fracture healing, the BMP-2 expression levels typically increase constantly until day 21, while VEGF expression peaks earlier. Therefore, they developed delivery systems that supported the early release of VEGF and the sustained release of BMP-2. In this work, Freeman et al. used multiple-tool biofabrication techniques to deliver VEGF and BMP-2 with distinct release profiles to improve regeneration of critically sized bone defects.
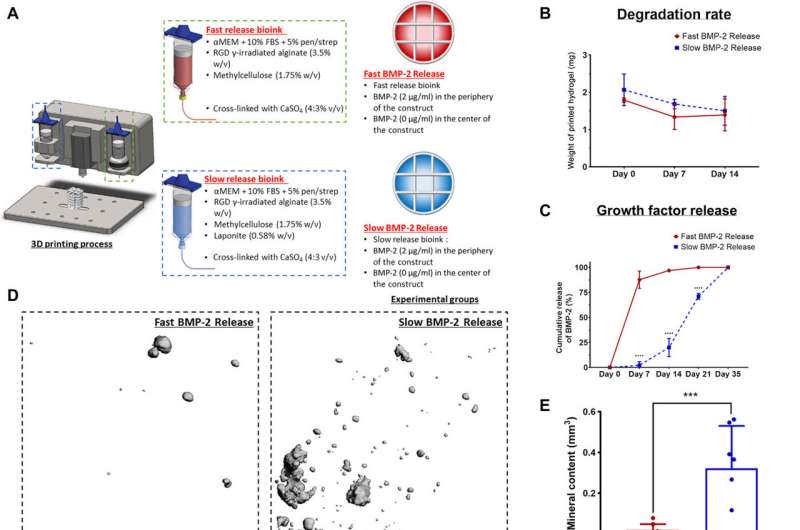
Localized-temporal delivery of BMP-2 led to controlled bone formation. (A) Schematic of the experimental groups. Construct design (4 mm in diameter, 5 mm in height). αMEM, alpha minimum essential medium. (B) Degradation of the two bioinks. (C) Cumulative release of BMP-2 of the fast release bioink versus the slow release bioink. (D) 3D reconstructions of the μCT data for each group at 8 weeks. (E) μCT analysis on total mineral deposition of each of the groups after 8 weeks in vivo. (F) μCT analysis on the location of mineral deposition of each of the groups after 8 weeks in vivo. ***P < 0.001; error bars denote SDs (n = 8 animals). (G) Goldner’s trichrome–stained sections of both groups after 8 weeks in vivo. Images were taken at 20×. White arrows denote developing bone tissue, and black arrows denote blood vessels. (H) Quantification of the amount of new bone formation per total area. Error bars denote SDs; **P < 0.01 (n = 8 animals, n = 6 slices per animal). Credit: Science Advances, doi:10.1126/sciadv.abb5093
Developing bioinks with distinct growth factor release profiles for in vivo implantation
The team controlled the release of morphogens (growth factors) from 3-D printed constructs, which they developed with alginate-based bioinks with different nanoparticles embedded to bind the regulatory factors. They slowed the release of BMP-2 in the constructs to enhance in vivo bone formation in predefined locations of the implant for accelerated bone healing while minimizing ectopic bone formation. Freeman et al. used clay nanoparticles or hydroxyapatite nanoparticles in the bioink made of alginate-methylcellulose to facilitate controlled VEGF release. They showed how the constructs maintained spatial gradients of growth factors for at least 14 days in the lab after bioprinting.
The printed VEGF constructs enhanced angiogenesis in vivo, which the team tested by depositing the constructs in 3-D printed polycaprolactone (PCL) implants to accelerate vascularization in a mouse model. Two weeks after subcutaneous implantation within the animal model, they conducted histological analyses to reveal the presence of vessels in homogenous and gradient VEGF groups. There were no obvious vessels present in the no VEGF group. Between two to four weeks, the gradient VEGF groups had significantly higher vessels in the periphery constraints, Freeman et al. noted the vessel growth in constructs to be mature and complete.
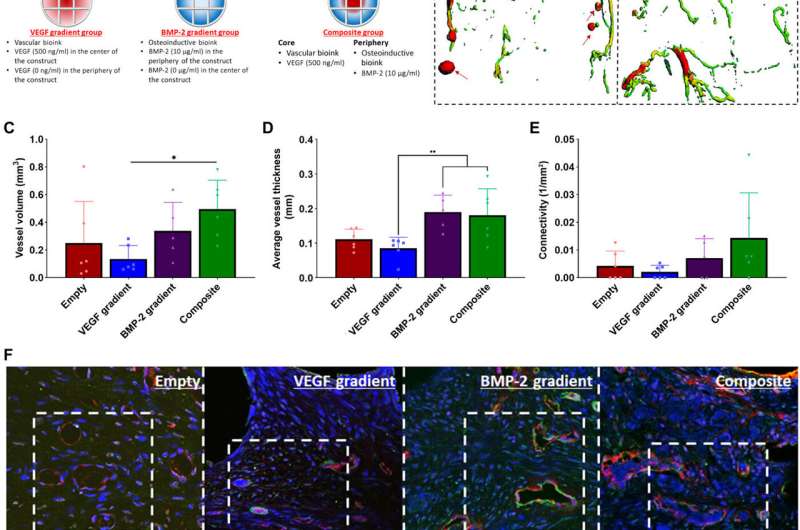
Spatiotemporal delivery of both VEGF and BMP-2 led to enhanced angiogenesis. (A) Schematic of the 3D printed experimental groups including key features of the developed bioinks and the segmental defect procedure. Construct design (4 mm in diameter, 5 mm in height). (B) μCT angiography representative images of vessel diameter. Red arrows denote leaky blood vessels denoted by pools of contrast agent. Quantification on (C) total vessel volume, (D) average vessel diameter, and (E) connectivity for all groups after 2 weeks in vivo. *P < 0.05 and **P < 0.01; error bars denote SDs (n = 9 animals). (F) Immunohistochemical staining of nuclei (blue), vWF (red), and α–SMA (green) of the experimental groups at 2 weeks after implantation. Images were taken at 40× and 63×. Yellow arrows denote vessels with α–SMA and vWF dual staining; white arrows denote slightly less mature vessels with only vWF positive staining. Credit: Science Advances, doi:10.1126/sciadv.abb5093
Delivering BMP-2 to localize bone formation in vivo
Since the slow and sustained release of BMP-2 is known to contribute to optimal bone regeneration, the team conducted bone formation studies in a mouse model with growth factor release within a distinct time-frame. To markedly reduce the release of BMP-2 from the bioink, Freeman et al. added laponite (a nanomedicine platform) and monitored its constant release from one to four weeks in the lab. To then understand the effects on bone formation (osteogenesis) in an animal model, the scientists constructed bioinks for fast BMP-2 release (without laponite) and slow BMP-2 release (with laponite) and mixed the bioinks with bone marrow-derived mesenchymal stem cells (BMSCs) and deposited them within 3-D printed scaffolds. They then implanted these scaffolds subcutaneously in the back of mice and followed the process by seeding additional mesenchymal stem cells to test bone growth in an ectopic location. Quantification studies showed the slow release of BMP-2 for significantly more new bone formation per total area of the construct. The work also showed the slow release of BMP-2 to have significantly more new bone forming potential per total area of the construct.
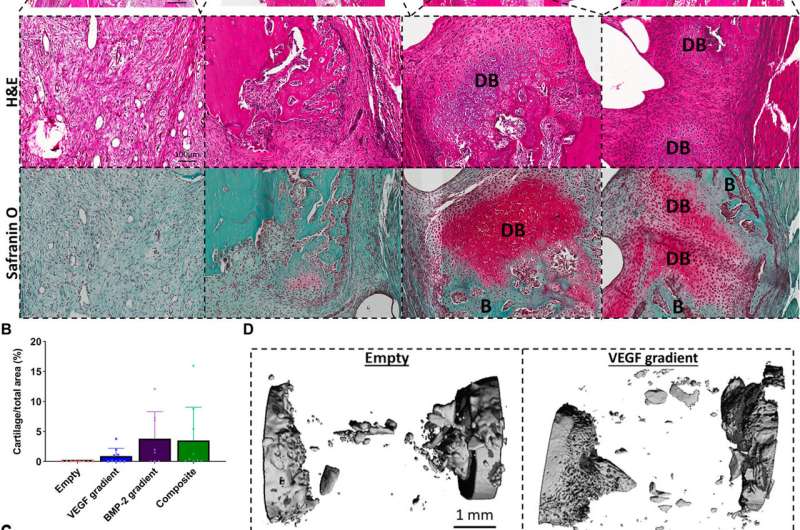
Temporal delivery of exogenous BMP-2 induces early bone healing via an endochondral ossification process. (A) Hematoxylin and eosin (H&E)- and Safranin O–stained sections of all groups after 2 weeks in vivo. Images were taken at 20×. DB denotes cartilage undergoing endochondral ossification to become developing bone, and B denotes positive new bone tissue. Quantification of the amount of (B) bone formation and (C) developing bone per total area. Error bars denote SDs (n = 9 animals). (D) μCT reconstructed images of the defect site. Credit: Science Advances, doi:10.1126/sciadv.abb5093
Enhancing angiogenesis within large bone defects
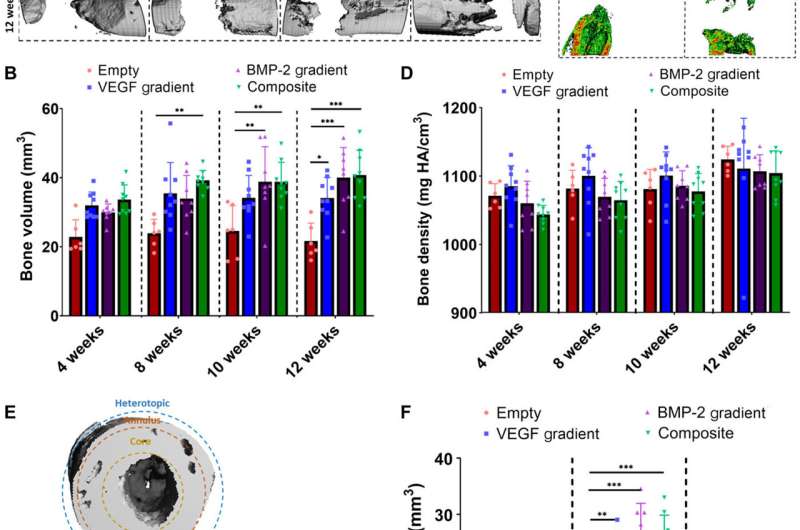
Freeman et al. next demonstrated the spatiotemporal delivery of growth factors (VEGF and BMP-2) to enhance angiogenesis. Using delayed-release BMP-2 constructs containing spatial gradients in VEGF, they recorded enhanced bone formation and blood vessel growth within critically sized rat femoral bone defects. Two weeks after implantation, micro-computed tomography (micro-CT) angiograms showed the appearance of vascular networks in all experimental groups used in the study, alongside primitive immature blood vessels with notable differences between different experimental constructs. Two weeks after surgery, they also noted positive staining for cartilage and new bone in BMP-2 gradient and composite experimental groups. When they conducted microCT tests to understand bone formation, they noted consistent healing patterns. Comparative bone density maps obtained within 12 weeks post-implantation revealed the new bones to contain cortical-like bone comparable to the adjacent native bone. To investigate heterotopic bone formation, the scientists conducted bone volume analysis in a region of interest on the 12 week of reconstruction, where bone preferentially formed in the annulus of the defect with little ectopic bone formation in all experimental groups. Additional histological staining revealed the predominant formation of fibrous tissue, bone marrow and cartilage.
Spatiotemporal delivery of both VEGF and BMP-2 led to enhanced bone tissue distribution 12 weeks after scaffold implantation. (A) Reconstructed in vivo μCT analysis of bone formation in the defects. (B) Quantification of total bone volume (mm3) in the defects at each time point. (C) Representative images of μCT bone densities in the defects at 12 weeks halfway through the defect (scale bar, 1 mm throughout). (D) Average bone density (mg HA/cm3) in the defects at each time point. (E) Outline of ROI bone volume analysis including definitions of core, annulus, and heterotopic regions. (F) Total bone volume (mm3) in each region at 12 weeks. **P < 0.01, ***P < 0.001, and ****P < 0.0001; error bars denote SDs (n = 9 animals). Credit: Science Advances, doi:10.1126/sciadv.abb5093
In this way, Fiona E. Freeman and colleagues developed 3-D printed implants with growth factors, which optimally included spatial gradients of VEGF coupled to BMP-2 to enhance large bone defect healing with little heterotopic (abnormal) bone formation. The team achieved an increased bone healing response using very low concentrations of exogenous growth factors. The next step will include translating such tissue engineering technologies from the bench to the bedside, which is a challenging, expensive and time-consuming process. The approach detailed in this work is an unprecedented strategy that allows spatiotemporal growth factor delivery to regenerate large bone defects or increase vascularization of any 3-D printed construct. The team envision future applications of this technology for controlled regeneration of diverse tissue types.
More information: Fiona E. Freeman et al. 3-D bioprinting spatiotemporally defined patterns of growth factors to tightly control tissue regeneration, Science Advances (2020). DOI: 10.1126/sciadv.abb5093
Michael Simons et al. Therapeutic angiogenesis in cardiovascular disease, Nature Reviews Drug Discovery (2003). DOI: 10.1038/nrd1226
Zarana S. Patel et al. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model, Bone (2008). DOI: 10.1016/j.bone.2008.06.019
Journal information: Science Advances , Nature Reviews Drug Discovery , Bone
© 2020 Science X Network