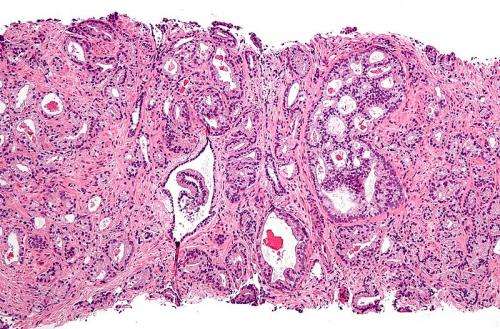
Micrograph showing prostatic acinar adenocarcinoma (the most common form of prostate cancer) Credit: Wikipedia, CC BY-SA 3.0
An antibody for treating advanced prostate cancer improves progression-free survival in patients with metastasised, castration-resistant prostate cancer. This is the finding of the long-term analyses of an international phase 3 clinical trial, recently published in the journal European Urology. The study showed that overall survival was two to three times higher than in the placebo arm.
Ipilimumab is a humanized monoclonal IgG1 antibody that is active against CTLA-4. CTLA-4 is a molecule that controls part of the immune system by down-regulating it. "Cancer cells can evade the endogenous defense of the immune system by deactivating it. An antibody that targets CTLA-4, a so-called checkpoint inhibitor (CPI), can block this deactivation, thereby reactivating the immune system once again. This reactivated immune response can then help the body to destroy cancer cells," explains oncologist Michael Krainer from the Department of Medicine I at MedUni Vienna/Vienna General Hospital and from the Comprehensive Cancer Center (CCC). The internationally renowned Urological Tumors working group from the division led by Krainer was invited to participate in the first global clinical phase 3 trial of a CPI in prostate cancer CA184-043, the long-term results of which have now been published in European Urology.
The recent trial included a total of 799 men. It was conducted globally, in the U.S., Canada, South America, Australia and in European countries. Patients were randomized in a 1:1 ratio to receive bone metastasis radiotherapy (a single 8 Gy fraction) followed by either ipilimumab 10 mg/kg or a placebo every three weeks via up to four injections. Although in the first planned analysis, the survival advantage in the treated group was present, it was not significant, whereas the recent analysis shows that long-term survival after three, four and five years is two to three times higher in the immunotherapy arm as opposed to the placebo arm.
Ipilimumab is licensed by the European Medicines Agency to treat melanoma, lung cancer and bladder cancer. However, there is still a lack of reliable data for approval to treat prostate cancer, since the first planned analysis did not show any significant survival advantage. In the light of the new long-term results, Krainer says, "Immunotherapy is highly promising and can be used, for example, when chemotherapy options have been exhausted or are undesirable. It can also be expedient to start it at an early stage, since any treatment is more effective if there is little cancer present and the patient is in good general health. We are the first group in Austria to gain such valuable experience and we are now attempting to incorporate immunotherapy into the treatment in the context of international clinical trials."
The working group will soon start on two study protocols using immunotherapy before a chemotherapy that is currently the standard treatment for patients with castration-resistant prostate cancer.
Prostate cancer is the second most frequent cancer in men worldwide. 365,000 men were diagnosed with prostate cancer in the E.U. in 2015. Patients with hormone-resistant prostate cancer need additional treatment options. According to US estimates, in five years from now, 10 to 20% of all prostate cancers will be castration resistant.
More information: Karim Fizazi et al. Final Analysis of the Ipilimumab Versus Placebo Following Radiotherapy Phase III Trial in Postdocetaxel Metastatic Castration-resistant Prostate Cancer Identifies an Excess of Long-term Survivors, European Urology (2020). DOI: 10.1016/j.eururo.2020.07.032
Journal information: European Urology
Provided by Medical University of Vienna