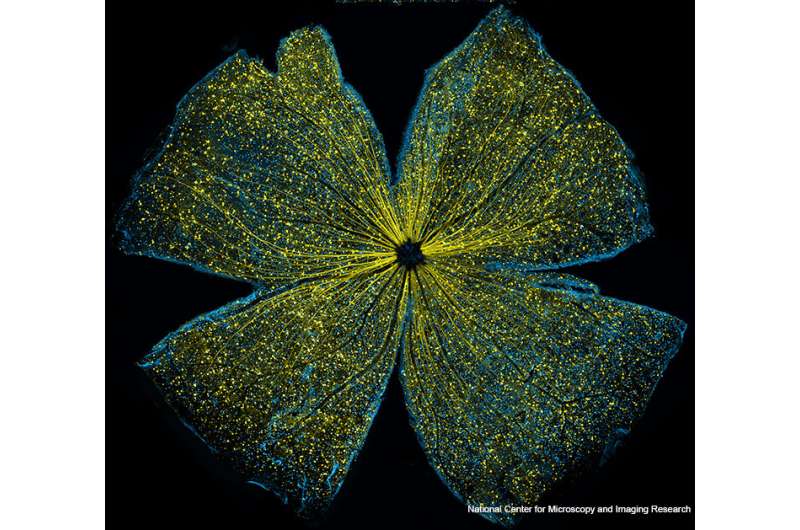
Mouse retina lg. Credit: National Center for Microscopy and Image Research
Research led by Jeff Gidday, Ph.D., Professor of Ophthalmology, Biochemistry, Neuroscience, and Physiology at LSU Health New Orleans School of Medicine, reports what is believed to be the first study in a mammalian model documenting the reprogramming of heritability to promote disease resilience in the next generation. The results are published in the Journal of Investigative Ophthalmology & Visual Sciences, the flagship journal of the Association for Research in Vision and Ophthalmology (ARVO).
The researchers used a functional measurement to document resilience to injury of the retina of adult mice that were born to parents who were exposed to intermittent, mild systemic hypoxia (reduced concentrations of oxygen) for several months prior to mating, even though the injury-protected mice received no treatment themselves. The therapy, akin to brief exposures to high-altitude air, is considered "epigenetic" because it modifies which genes are converted into proteins in tissues throughout the body, including germ cells (sperm and eggs).
"We exposed mice to nonharmful hypoxia to trigger these adaptive changes" says first-author Jarrod Harman, a doctoral student in Dr. Gidday's lab. "But there are many epigenetic stimuli that could cause these changes as well, including exercise, and other 'positive' stressors. Not all stress is bad for you."
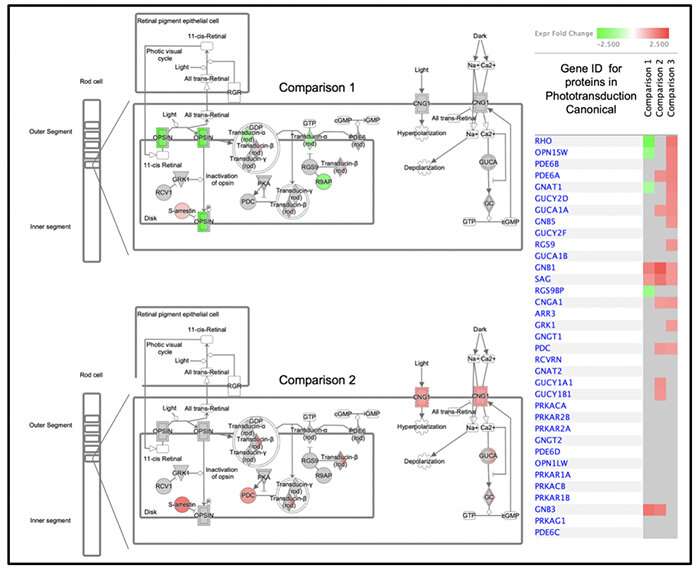
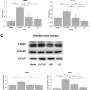
The researchers also extensively analyzed the injury-resilient retinae. By comparing the protein profiles of these retinae to those of mice derived from untreated parents using mass spectrometry, they identified many of the potential proteins and related biochemical mechanisms by which this intergenerational, injury-resilient state is achieved. These proteins, serving in both functional and structural roles, represent potential therapeutic targets for drug development to protect against retinal diseases associated with an inadequate blood supply (ischemic retinopathies).
Gidday table lg. Credit: Louisiana State University
Other studies have shown that repetitive exposure to adverse stimuli can enhance the susceptibility of first-generation offspring to disease. But Gidday contends that, conversely, this is the first study to use a mild, nonharmful stress like intermittent systemic hypoxia to provide protection against disease in first-generation offspring.
"Research has shown that environmental enrichment can enhance some baseline memory metrics in both parents and offspring," Gidday says, "but no study has ever shown that offspring can inherit a neuroprotective phenotype induced in parents by epigenetics. The implications of this finding with respect to our understanding of the heritability of disease susceptibility, and disease resilience, is profound."
Companion studies addressing the safety profile of the treatment showed that the intermittently reduced oxygen stimulus used to trigger injury resilience across generations caused no injury to the most oxygen-sensitive cells of the brains of mice receiving the treatment, nor affected the normal structure or function of the retina of the adult offspring derived from treated mice.
Ischemic retinopathies are diseases that result from some degree of prolonged, lower-than-normal blood flow to the retina, compromising the delivery of much-needed oxygen and glucose to this very metabolically active tissue. The most well-known examples are glaucoma and diabetic retinopathy, but the retina can also be deprived of blood flow acutely, as happens with a heart attack or stroke. Collectively, the resultant visual impairment suffered by those with these retinopathies is staggering, and in a large percentage of cases, complete blindness can ensue.
"The direct inheritance of an induced phenotype is what Lamarck famously proposed in 1809, the year Darwin was born" says Gidday. "Here we are, almost 200 years later, finding evidence to support this concept, despite it being largely displaced for the last 150 years by Darwin's Theory of Natural Selection. More than likely both operate under distinct environmental situations to enhance both short- and long-term reproductive fitness."
More information: Jarrod C. Harman et al, Intermittent Hypoxia Promotes Functional Neuroprotection from Retinal Ischemia in Untreated First-Generation Offspring: Proteomic Mechanistic Insights, Investigative Opthalmology & Visual Science (2020). DOI: 10.1167/iovs.61.11.15
Provided by Louisiana State University