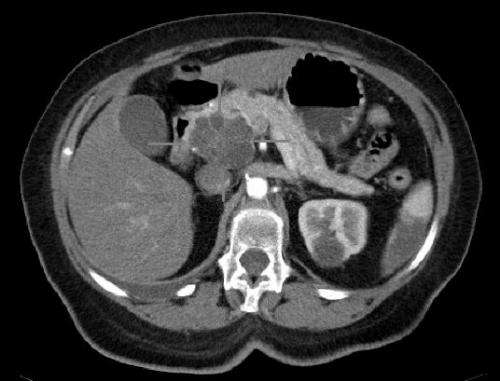
Axial CT image with i.v. contrast. Macrocystic adenocarcinoma of the pancreatic head. Credit: public domain
A team of researchers led by Mayo Clinic and the Translational Genomics Research Institute (TGen), an affiliate of City of Hope, has identified specific potential therapeutic targets for the most aggressive and lethal form of pancreatic cancer.
In what is believed to be the most comprehensive analysis of adenosquamous cancer of the pancreas (ASCP), the Mayo Clinic and TGen team identified, in preclinical models, therapeutic targets for this extremely fast-moving and deadly form of pancreatic cancer, and identified already available cancer inhibitors originally designed for other types of cancer, according to a study published today in Cancer Research, a journal of the American Association for Cancer Research (AACR).
"The rarity of ASCP, the scarcity of tissue samples suitable for high resolution genomic analyses, and the lack of validated preclinical models, has limited the study of this particularly deadly subtype of pancreatic cancer," said Dr. Daniel Von Hoff, Distinguished Professor and TGen's Physician-In-Chief, considered one of the nation's foremost authorities on pancreatic cancer, and one of the study's authors. "We need entirely new possible approaches for our patients with ASCP."
Pancreatic ductal adenocarcinoma (PDAC) is the most common form of pancreatic cancer, which this year is projected to kill nearly 57,600 Americans, making it the nation's third leading cause of cancer-related death, according to the American Cancer Society. Among pancreatic cancer patients, a small percentage (less than 4%) are diagnosed with ASCP, a particularly aggressive form of pancreatic cancer.
"ASCP currently has no effective therapies. Unlike PDAC, ASCP is defined by the presence of more than 30% squamous (skin-like) epithelial cells in the tumor. The normal pancreas does not contain squamous cells," said the study's senior author, Michael Barrett, Ph.D., who holds a joint research appointment at Mayo Clinic and TGEN.
"Our study has shown that ASCPs have novel 'hits' (mutations and deletions) in genes that regulate tissue development and growth superimposed on the common mutational 'landscape' of a typical PDAC. As a consequence, cells within the tumor have the ability to revert to a stem-cell-like state that includes changes in cell types and appearance, and the activation of signaling pathways that drive the aggressive nature of ASCP,' said Dr. Barrett.
While this activated aggressive stem-cell-like state is very resistant to current therapies for pancreatic cancer, Dr. Barrett said, the study has shown that ASCP can be targeted by drugs currently in clinical use for other cancers as well as non-cancer related conditions.
Using multiple cancer analysis methods and platforms—including: flow cytometry, copy number analysis, whole exome sequencing, variant calling and annotation, ATAC-seq, immunofluorescence, immunohistochemistry, single cell sequencing, and organoid cultures and treatments—the Mayo Clinic and TGen research team conducted what is believed to be the most in-depth analysis of ASCP tissue samples.
Researchers identified multiple mutations and genomic variants that are common to both PDAC and the more aggressive ASCP. "Of significant interest," the study says, the team also identified two potential therapeutic targets unique to ASCP genomes: FGFR signaling, including an FGFR1-ERLIN2 gene fusion, and a pancreatic cancer stem cell regulator known as RORC.
These data provide a unique description of the ASCP genomic and epigenomic landscape and identify candidate therapeutic targets for this lethal cancer, the study says.
Using organoids, which are laboratory cultures derived from samples of patient tumors, researchers tested the activity and functional significance of candidate therapeutic targets. According to the study: "Specifically, organoids carrying the FGFR1-ERLIN2 fusion show a significant response to pharmacological FGFR inhibition," providing new candidate targets for developing therapies for patients with ASCP.
In addition, the study says, "To our knowledge, this is the first study to apply DNA content sorting to the genomic analysis of ASCP," a method that purifies the cancer DNA from other cells and parts of cells, thereby eliminating any biological "noise" that might impede the precision of the analysis.
Using an interrogation tool known as ATAC-seq, researchers also identified RORC as another distinguishing feature of ASCP.
"Of significant interest will be clinical trials with FGFR and RORC inhibitors that include correlative studies of genomic and epigenomic lesions in both ASCP and PDAC," the study concludes.
Also contributing to this study—Genomic and Epigenomic Landscaping Defines New Therapeutic Targets for Adenosquamous Carcinoma of the Pancreas—were: Virginia G Piper Cancer Center at HonorHealth; University of California, San Diego School of Medicine; Salk Institute for Biological Studies; Memorial Sloan Kettering Institute; and University of Nebraska Medical Center (UNMC).
More information: Elizabeth Lenkiewicz et al, Genomic and Epigenomic Landscaping Defines New Therapeutic Targets for Adenosquamous Carcinoma of the Pancreas, Cancer Research (2020). DOI: 10.1158/0008-5472.CAN-20-0078
Journal information: Cancer Research
Provided by The Translational Genomics Research Institute