18F-Fluciclovine PET/MRI for prostate cancer staging, androgen deprivation evaluation
According to an open-access article in ARRS' American Journal of Roentgenology (AJR), fluorine-18-labeled fluciclovine PET/MRI demonstrated utility in the initial staging of high-risk prostate cancer, as well as for evaluating the response to androgen deprivation therapy (ADT).
Between January 2018 and February 2019, 14 men with newly diagnosed high-risk prostate cancer and negative or equivocal conventional staging imaging were enrolled in this prospective pilot study (ClinicalTrials.gov registration number NCT03264456). "All patients underwent pretreatment 18F-fluciclovine PET/MRI including multiparametric prostate MRI; twelve underwent 18F-fluciclovine PET/MRI after surgery or between ADT and radiotherapy," explained lead investigator Samuel Joseph Galgano from the University of Alabama at Birmingham's multidepartment team.
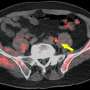
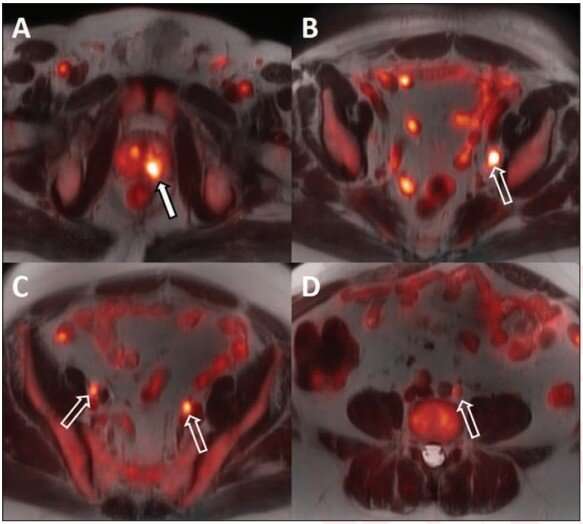
For all 14 patients, the biopsy-proven lesion in the prostate gland was accurately identified on both MRI and 18F-fluciclovine PET/MRI, and the activity noted on PET/MRI correlated with both the MRI-defined intraprostatic lesions and biopsy-proven prostate cancer. Suspected pelvic regional nodal metastases were detected in 3 patients on MRI vs 7 patients on PET/MRI. Of the three patients with suspected nodal metastases on MRI, 18F-fluciclovine PET/MRI was concordant for lymph node metastases and demonstrated additional suspected lymph node metastases not detected on MRI alone.
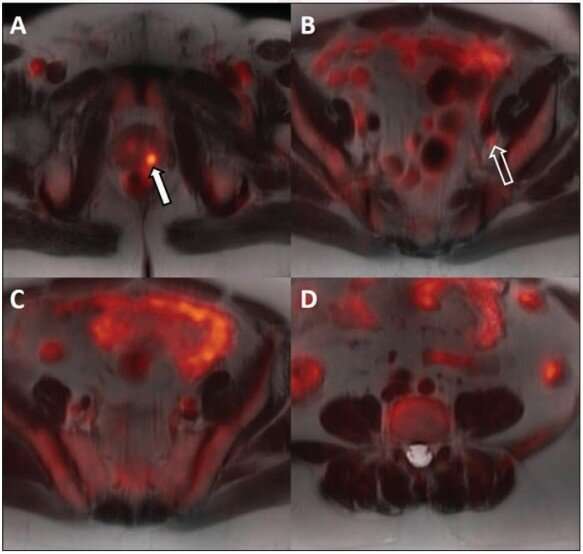
Ten of the 14 patients underwent ADT prior to radiation therapy. The primary intraprostatic lesion was accurately identified in all 10 patients, 7 of whom demonstrated suspicious lymph nodes on the pretreatment PET/MRI. Following ADT, all 10 patients demonstrated a decrease in tracer activity either with the primary intraprostatic lesion, all suspicious lymph nodes detected on the pretreatment 18F-fluciclovine PET/MRI, or both.
"Given the FDA approval and widespread availability of 18F-fluciclovine," the authors of this AJR article concluded, "the findings could have impact in the immediate future in guiding initial management of patients with prostate cancer."
More information: Samuel Joseph Galgano et al, Utility of 18F-Fluciclovine PET/MRI for Staging Newly Diagnosed High-Risk Prostate Cancer and Evaluating Response to Initial Androgen Deprivation Therapy: A Prospective Single-Arm Pilot Study, American Journal of Roentgenology (2020). DOI: 10.2214/AJR.20.24509