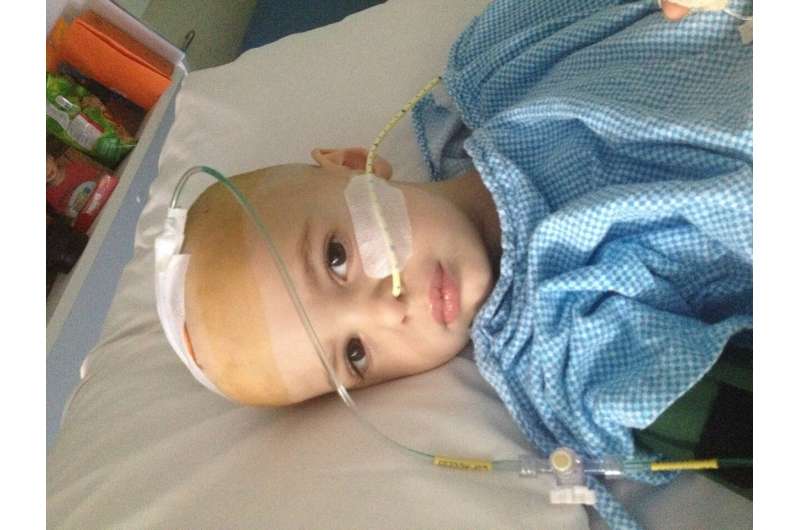
Thomas Edney was 2.5 when he was admitted into hospital with tumors in his brain and spinal cord. Multiple diagnoses and a failure to improve led him to the ZERO program whichshowed a mutation believed to be driving Thomas' cancer. The ZERO team was able tofind a therapy capable of targeting the mutation. In November 2019, Thomas was able to access a pediatric clinicaltrial using a gene therapy drug called Afatinib. Thomas's acceptance into the trial was only possible due to the ZEROprogram which included personalized analysis of Thomas' tumor sample.In January 2020, after two months of treatment, Thomas' brain scan showed a noticeable improvement. Today, Thomas is eight years old and still receiving the therapy. His tumour has shrunk, and he is no longer indebilitating pain. Credit: Children's Cancer Institute
The success of an Australian program which aims to find the most-likely-to-succeed personalized treatment recommendations for children with high risk cancer has been revealed in a paper published today 6 October in the prestigious journal, Nature Medicine.
The program uses state of the art genetic technology to test each child's tumor with a comprehensive suite of genetic tests to find a therapy that can potentially treat the cancer. This testing is completed within a clinically relevant timeframe of under 9 weeks on average, with more than 70% of the children being recommended a personalized treatment program. Of these cases, at least one, and sometimes several, genetic changes are identified that lead to the potential treatment recommendations.
The paper's description of the Zero Childhood Cancer Personalized Medicine Program (ZERO), led by Children's Cancer Institute and Kids Cancer Centre at Sydney Children's Hospital, Randwick—offers a new paradigm for the management of pediatric cancers with global implications.
Every year close to 1000 children and young people in Australia are diagnosed with cancer, and of these, three children die each week. The challenge in curing every child of cancer is that every child and every cancer is unique—which is why the current standard treatment options don't always work.
One of the world's most comprehensive personalized medicine programs for children and young people with cancer, ZERO aims to identify the precise make-up of each child's cancer by using sophisticated genomic tests (whole genome sequencing, whole transcriptome sequencing and methylation profiling) to identify the molecular changes (including "mutations") driving their cancer, and matching these with treatments most likely to target their unique cancer. The aim is to identify a personalized treatment plan for each child.
Zero Childhood Cancer researchers, Associate Professors Paul Ekert, Mark Cowley, and David Ziegler, with the support of a vast team of researchers and clinicians, studied the genomic data generated from the first 250 children enrolled on the program and have shown that ZERO identified the molecular, or genetic, basis of a child's cancer in more than 90% of cases and 70% had at least one new potential treatment option identified based on their cancer's genetic makeup.
At the time of publishing this report, 32% of children for whom a therapeutic recommendation was made, had received the recommended therapy. The early results of those children showed that in 30% of cases the tumor shrank, and in some patients completely regressed. In another 40% of cases the tumor stopped growing and stabilized.
According to Associate Professor Ziegler, Pediatric Oncologist, Co-Chair, ZERO's National Clinical Trial, Head, Clinical Trials Unit, Kids Cancer Centre, Sydney Children's Hospital, Randwick and Brain Tumours Group Leader, Children's Cancer Institute, "these are very optimistic early results for children with the highest risk cancers," he said. "We have shown that ZERO has the potential to find personalized, novel treatment strategies for children with the most aggressive cancers who in many cases have no other treatment options available. Patients who received the molecularly informed, recommended therapy had a much higher rate of clinical benefit than we would expect from non-targeted approaches."
Associate Professor Cowley, WGS Lead, ZERO, Computational Biology Group Leader, Children's Cancer Institute said the success of the ZERO program, reported today, represents a paradigm shift in the way high risk childhood cancer can be treated. "We have developed a highly effective approach for molecularly profiling a child's cancer and extracting the medically relevant information from this complex data in a clinically relevant timeframe," he said. "We have shown that this approach informs patient diagnosis, identifies new potential treatment options, and identifies families that may be at higher risk of developing additional childhood cancers."
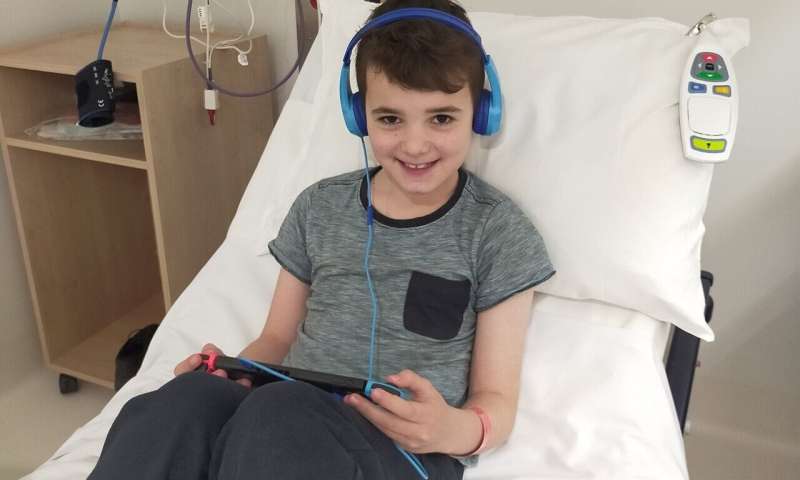
This photo - taken earlier this year - shows Thomas after starting a trial with a drug targeted at his tumor's genetic makeup. His cancer has shrunk and he is without pain for the first time in over 6 years. Credit: Children's Cancer Institute
According to Associate Professor Ekert, 'Omics Lead, ZERO, Co-Head of Theme, Personalized Medicine, Translational Tumor Biology, Group Leader, Children's Cancer Institute, the fact that in more than 90% of cases the genetic cause of the cancer was identified is important as it provides a way to systematically identify the molecular basis of most childhood cancers. "We are now able to prise open the lid of the black box of this previously poorly understood collection of diseases," he said.
ZERO has helped children like Thomas, who was diagnosed at age 2 and a half, with a rare form of brain cancer. Thomas' condition was deteriorating, and after undergoing multiple surgeries and losing much of his eyesight, the treatment options were quickly running out. Once Thomas was enrolled on ZERO, a rare mutation driving his cancer growth was found, and a new experimental drug was found to target it. He had a dramatic response to this treatment with shrinkage of his tumor. Today Thomas is 8 years old and whilst he is still receiving his therapy, his future looks bright—he is back at school and able to return to the activities he enjoys.
However, this long-term outcome is not yet the case for all children with cancer. Three-year-old Stella suffered with an extremely aggressive brain cancer that meant she could no longer talk, walk or eat unaided and was in palliative care when she was enrolled on the ZERO program in 2017. A genetic driver was identified in her brain tumor, and when she received the recommended targeted therapy her tumor completely disappeared, she was well enough to run around and play like any healthy child. However sadly, after 10 months of treatment her tumor grew back and she passed away on 1 August 2018. While ZERO could not save Stella, it was able prolong her life, giving her 10 months of happy and relatively healthy time with her family, that they otherwise would not have had.
ZERO is changing the model of care for children with cancer by identifying new treatments specifically targeted to each child's individual cancer and facilitating access to the right drug for the right child at the right time.
Thanks to $67 million in new funding announced in April 2020 from the Australian Government's Medical Research Future Fund and Minderoo Foundation to extend and scale the ZERO program, the potential of these promising early results could benefit up to 1,000 children and young people per year by end 2023.
More information: Whole genome, transcriptome and methylome profiling enhances actionable target discovery in high-risk pediatric cancer, Nature Medicine (2020). DOI: 10.1038/s41591-020-1072-4 , www.nature.com/articles/s41591-020-1072-4
Journal information: Nature Medicine
Provided by Children's Cancer Institute Australia