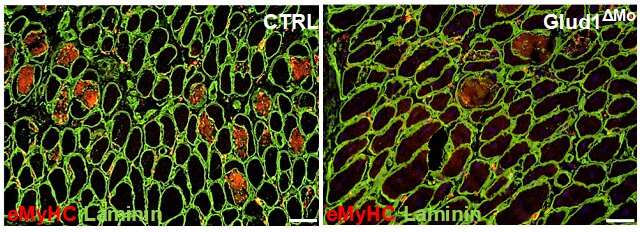
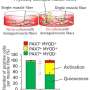
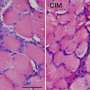
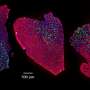
Representative images of embryonic myosin heavy chain (eMyHC) expression in TA muscle 6 days after CTX injury. Credit: VIB (the Flanders Institute for Biotechnology)
A team headed by Prof. Massimiliano Mazzone (VIB-KU Leuven Center for Cancer Biology), in collaboration with Dr. Emanuele Berardi and Dr. Min Shang, revealed a new metabolic dialogue between inflammatory cells and muscle stem cells. The researchers show that strengthening this metabolic crosstalk with an inhibitor of the enzyme GLUD1 fosters the release of glutamine, and improves muscle regeneration and physical performance in experimental models of muscle degeneration such as trauma, ischemia, and aging. Besides its translational potential, this work also provides key advances in several fields of research including muscle biology, immunometabolism, and stem cell biology.
The role of glutamine
Skeletal muscle is instrumental to move our body, but it is also a large reservoir of amino acids stored as proteins and it influences energy and protein metabolism throughout the human body. The role of the amino acid glutamine has been considered central for muscle metabolism because of its abundance. However, its precise role after trauma or during chronic muscle degenerative conditions were largely neglected.
The team of Prof. Massimiliano Mazzone (VIB-KU Leuven Center for Cancer Biology) observed that, upon damage or during aging, the normal levels of glutamine in the muscle decrease as a consequence of dead muscle tissue. The researchers identified a metabolic dialogue between the inflammatory cells arriving after injury, and the resident muscle stem cells. This cellular crosstalk re-establishes the original levels of glutamine in the muscle and, in doing so, it prompts the regeneration of the muscle fibers.
Regenerating muscle
Using in vitro and in vivo state-of-the-art methodologies the researchers showed that muscle injury, ischemia and aged-related muscle wasting are conditions characterized by reduced glutamine. One reason for this is the loss of glutamine production by the muscle itself because of its damage.
Dr. Berardi explains, "Using genetic tools and pharmacological drugs inhibiting GLUD1, an enzyme that metabolizes glutamine, we could prevent the post-injury drop in glutamine. This resulted in the overproduction of glutamine by inflammatory cells, named macrophages, reaching the muscle after damage. The newly produced glutamine could be used by muscle stem cells to quickly regenerate the damaged muscle tissue. We found the same thing in acute as well as chronic degenerative conditions, such as aging, leading to a faster muscle functional recovery."
Preventing degeneration
This study reveals glutamine as a sensor molecule whose levels in the muscle tissue control a regenerative program and suggests that GLUD1 is a therapeutic target that could enable opportunities for muscle regeneration after acute injury or chronic degenerative conditions such as aging.
Prof. Mazzone emphasizes the potential of their discovery: "This provides hope for the treatment of degenerative muscle conditions, including trauma, ischemia and aging. The latter in particular represents an important challenge for healthcare with an increasing average age of the global population, accompanied by aged-related sarcopenia. In a joined effort with VIB Discovery Sciences and the Centre for Drug Design and Discovery, we are currently developing and testing more selective GLUD1 inhibitors for chronic muscle wasting conditions including muscular dystrophy."
More information: Min Shang et al. Macrophage-derived glutamine boosts satellite cells and muscle regeneration, Nature (2020). DOI: 10.1038/s41586-020-2857-9
Journal information: Nature
Provided by VIB (the Flanders Institute for Biotechnology)