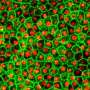
Captured by the lab of Jesse Schallek, assistant professor of ophthalmology and neuroscience, the image shows microscopic immune cells escaping a nearby blood vessel in response to inflammation. The color overlay shows computer detection of single cells that are tracked over time. Credit: Schallek lab
University of Rochester vision scientist Jesse Schallek can barely contain his excitement as he shares time-lapse videos showing immune cells moving through living retinal tissue at the back of an eye.
In one clip, immune cells crawl so slowly along the inside edge of a blood vessel that the video must be sped up 25 times to show their progress. Another cell slowly treads against the flow of blood in a vessel, like a salmon fighting its way upstream. Other immune cells leave the blood vessels and inch through the surrounding tissue, then congregate in a swarm, forming a beehive of activity.
Schallek and his vision lab at the University of Rochester Center for Visual Science and Flaum Eye Institute, have created a new microscopy technique, described in the journal eLIFE, that builds upon groundbreaking adaptive optics developed at the University more than 20 years ago.
Combined with time lapse videography and artificial intelligence software, the new technique enables researchers for the first time to noninvasively image and track—without labeling—the interactions of translucent immune cells within live retinal tissue in animals. Until now, the immune cells had to be labeled with fluorescent agents and often reinjected in order to image them—raising questions about how this might change the behavior of the cells. Another common, but limiting approach is to remove cells and study them with a microscope in a dish.
Schallek's lab avoids both complications by imaging immune cells in the living eye without requiring dyes at all—the first study of its kind to do so.
"We think of the eye as this beautiful window where we can peer in, noninvasively, without having to cut or insert a camera into places where we would rather it didn't go," says Schallek, an assistant professor of ophthalmology and neuroscience.
"The eye is an extension of our brain, and therefore with this technology we have some of our first glimpses into immune cell function deep in the central nervous system. This is a critical step forward for basic science and clinical study alike."
Schallek's lab is now adapting the new technique for use with human patients.
"We think this will be a game changer for ophthalmology and for our understanding of retinal diseases that lead to blindness," says Schallek.
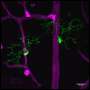
Using the new techique, researchers in the lab of Rochester vision scientist Jesse Shallek captured images of the same retinal location and tracked it over two months as the area responded to injury. Single immune cells arrive and respond with vigor at 6 hours and 24 hours after the injury. The retina then returns to normal conditions after weeks to months. Credit: Schallek lab
What role do immune cells play in inflammation?
Immune cells are at the center of "a whole cascade of events" that cause the inflammation that is characteristic of most retinal eye diseases that lead to blindness, Schallek says. For example, in addition to immune cells arriving at the affected tissue, and releasing compounds that recruit more immune cells, there are also changes in blood flow—all of which interfere with vision and complicate the progression of the disease.
Until now, the tools available to measure inflammation in retinal tissue have been limited.
Optical coherence tomography, for example, has been used to measure the thickness of retinal tissue at the back of the eye. "The thickness of that tissue is thought to be a marker for how inflamed the tissue is," Schallek says. "However useful that might be, it doesn't really tell you what the cells in that tissue are doing."
The new technology developed by his lab does that by:
- Building on the adaptive optics technology created at Rochester by David Williams, director of the Center for Visual Science and his colleagues more than 20 years ago. Adaptive optics provided a way to correct for aberrations of the eye so that researchers for the first time could visualize individual cells at the back of the eye.
- Integrating into adaptive optics a new phase contrast technique—much like differential interference contrast microscopy–which can capture images of translucent objects such as immune cells.
- Using time lapsed videography to capture images of immune cell activity in the retina over periods ranging from milliseconds to months. Adjusting the playback speed allows the slow movements of those cells can be more easily tracked.
- Using artificial intelligence (AI) computer code deployed by the lab to identify the different kinds of immune cells captured in the images.
- Using ultra-high-speed imaging of individual red blood cells to simultaneously track blood flow and how it changes in response to the inflammation.
The study was led by first authors Aby Joseph, a Ph.D. candidate at the Institute of Optics and Colin Chu, an ophthalmologist and visiting senior research fellow from University of Bristol who spent three months in Schallek's lab.
"This was a wonderful collaboration, merging expertise in immunology and cutting-edge imaging technology," says Chu. "A defining characteristic of immune cells is that they are truly mobile and incredibly dynamic, rushing to wherever inflammation occurs. The first time we successfully imaged them was astounding, as we were essentially spying on them as they worked within their actual native setting. Even now watching the recordings continues to mesmerize me."
Using his optics background, Joseph added key engineering advances rendering immune cell imaging and blood flow quantification in the retina. "The use of low levels of infrared light to achieve this means that our approach can be safely translated to human study," says Joseph.
"Additionally, the use of high-speed imaging to measure blood flow revealed surprising details about how inflammation behaves in the central nervous system. We found that veins and arteries achieve an increase in blood flow to the inflamed retina through markedly different ways. This could become important for designing and testing future treatments to resolve inflammation," says Joseph.
This new technology is exciting not only from a scientific and clinical standpoint, but especially for pharmaceutical applications, Schallek says. "Companies will now have way to look at how well specific drugs target specific components within the immune system. They'll be able to see if they can improve the efficacy of drugs that are already approved, and others that are still in development."
More information: Aby Joseph et al. Imaging single-cell blood flow in the smallest to largest vessels in the living retina, eLife (2019). DOI: 10.7554/eLife.45077
Provided by University of Rochester