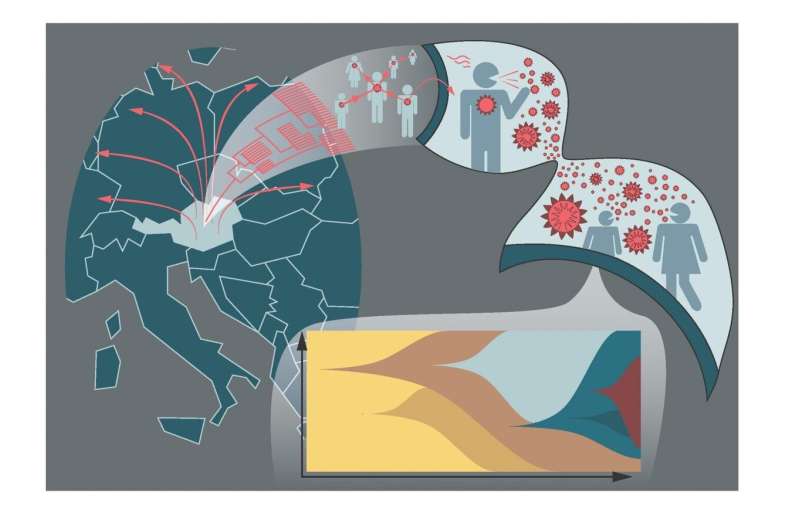
Austrian study reveals important characteristics of the virus: The analysis of infection clusters and superspreading events in Austria built the foundation to obtain general information on transmission properties between persons and the mutation of the virus in patients. Credit: Andreas Bergthaler's Group / CeMM
In the COVID-19 pandemic, 57 million people have already been infected worldwide. In the search for vaccines and therapies, a precise understanding of the virus, its mutations and transmission mechanisms is crucial. A recent study by the research group of Principal Investigator Andreas Bergthaler at the CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences, in the renowned journal Science Translational Medicine, makes an important contribution to this. The high quality of epidemiological data in Austria, together with state-of-the-art virus genome sequencing, has supported unprecedented insights of the mutation behavior and transmission of the SARS-CoV-2 virus.
The project "Mutational dynamics of SARS-CoV-2 in Austria" was launched by CeMM in close cooperation with the Medical University of Vienna at the end of March. Together with the Austrian Agency for Health and Food Safety (AGES) and in cooperation with numerous universities and hospitals all over Austria, scientists are working on drawing a more precise picture of the virus mutations and transmissions that occur by genome sequencing of SARS-CoV-2 viruses. Under the leadership of CeMM Principal Investigators Andreas Bergthaler and Christoph Bock, 750 samples from important SARS-CoV-2 infection clusters in Austria such as the tourist town of Ischgl and Vienna were phylogenetically and epidemiologically reconstructed and their role in transcontinental virus spread was analyzed. The results also provide important information on transmission and the development of mutations in the SARS-CoV-2 virus.
Mutation analyses revealed correlations between clusters
Based on epidemiological data, the scientists used mutation analyses to reconstruct a SARS-CoV-2 cluster consisting of 76 cases and to uncover a cryptic link between two epidemiological clusters. "This example illustrates how contact tracing and virus mutation analysis together provide a strong pillar of modern pandemic control," says project leader Andreas Bergthaler. Franz Allerberger, Head of the Public Health Division of AGES and co-author of the study, agrees: "The modern techniques of virus genome sequencing support epidemiological contact tracing and offer high-resolution insights of the ongoing pandemic."
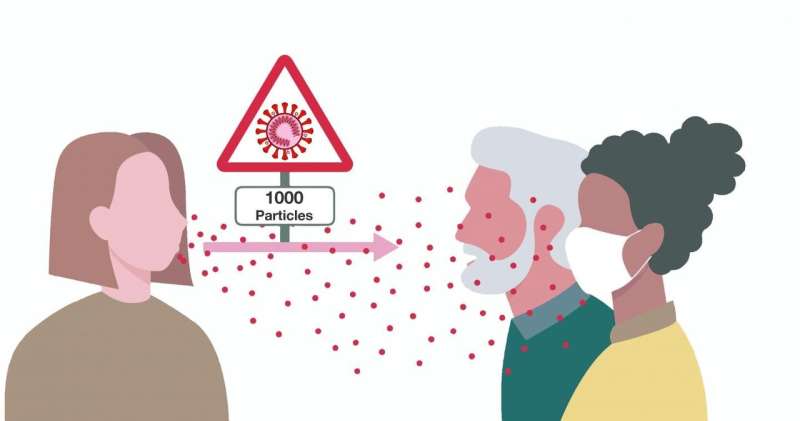
The analysis of epidemiologically-validated chains of infections in Austrian superspreading events found that a relatively large average dose of 1000 infectious viral particles is transmitted. Credit: CeMM
Researchers observe the development of new mutations
A special feature of the study is that a chain of eight consecutive transmissions was analyzed. "The transmission chain started with a returnee from Italy. Within 24 days, the SARS-CoV-2 virus spread in the greater Vienna region via public and social events in closed rooms", say the CeMM study authors Alexandra Popa and Jakob-Wendelin Genger. The precise breakdown of the transmission chain enabled the scientists to closely observe the development of a new mutation of SARS-CoV-2. "Thanks to excellent epidemiological and our deep virus sequencing data, we could follow how the SARS-CoV-2 virus mutated in one individual and was then transmitted to others," explains Andreas Bergthaler. In addition, the scientists observed the mutation behavior of the virus during the course of the disease in 31 patients. This may help in the future to assess whether treatments influence the mutation characteristics of the virus.
On average 1,000 virus particles are transmitted during an infection
The results of the current analyses also show that on average 1000 infectious virus particles are transmitted from one infected person to the next. These values are overall considerably higher than for other viruses such as HIV or noroviruses. Andreas Bergthaler adds: "Yet, occasionally we also found infected people who apparently came into contact with fewer virus particles and still became infected. We suspect that parameters such as the application of protective measures, the transmission route or the immune system may play a decisive role here." These results raise important new questions and hypotheses. Reducing the viral load of infected individuals by a combination of measures such as mouth-nose protection, physical distance and adequate indoor air exchange could play a key role in both preventing the spread of the virus and possibly even influence the course of the disease.
The current study based on data collected during the early phase of the SARS-CoV-2 pandemic in spring 2020, provides important insights into the fundamental dynamics of SARS-CoV-2 mutations within patients and during transmission events. These results support other ongoing research projects aiming at a better understanding and controlling the pandemic.
More information: Alexandra Popa et al, "Genomic epidemiology of superspreading events reveals mutational dynamics and transmission properties of SARS-CoV-2", Science Translational Medicine on 23 November 2020. DOI: 10.1126/science.abe2555
Journal information: Science Translational Medicine
Provided by Austrian Academy of Sciences