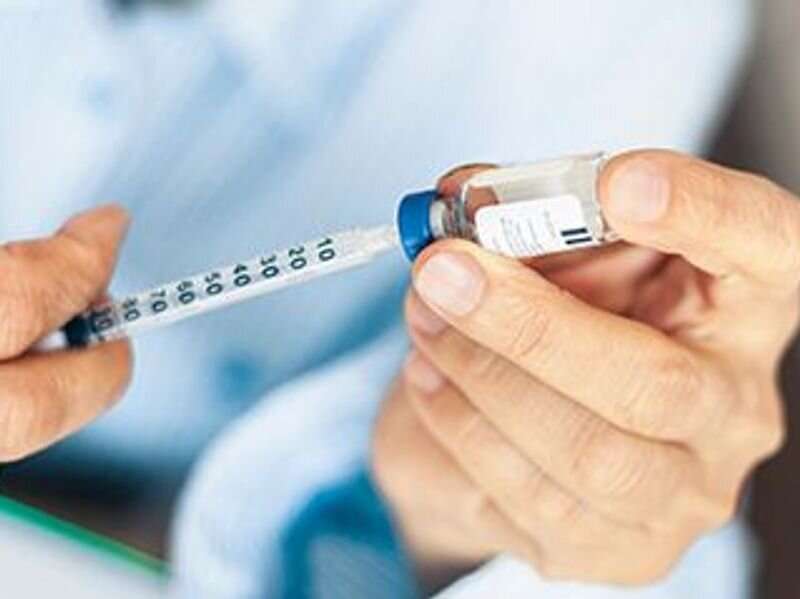
(HealthDay)—When queried in polls conducted earlier this year, only about half of American adults said they planned to get any vaccine against the new coronavirus. But after a largely successful rollout this month of two safe and effective shots, many of those initial doubters now say they'll line up to get their vaccine doses when their turn comes.
According to The New York Times, polls conducted by Gallup, the Kaiser Family Foundation and the Pew Research Center all show vaccine acceptance rates rising from about 50% this summer to more than 60% and, in one poll, 73%.
That last number approaches the threshold scientists have deemed necessary for herd immunity, where enough of a population is immune and the spread of the coronavirus begins to recede.
"As soon as it is my turn to get the vaccine, I will be there front and center! I am very excited and hopeful," Joanne Barnes, 68, a retired elementary school teacher from Fairbanks, Alaska, told the Times.
Earlier this summer, Barnes had told the paper the opposite; that she would not get the shot. The game-changers for her, Barnes said, were "the Biden administration, returning to listening to science and the fantastic stats associated with the vaccines."
With more than 19 million COVID-19 cases in the United States by Monday and more than 333,000 Americans now killed by the disease, more people than ever have now been personally affected by the new coronavirus. That harsh reality might also be driving some to reconsider getting the shot.
"More people have either been affected or infected by COVID," Rupali Limaye, an expert on vaccine behavior at the Johns Hopkins Bloomberg School of Public Health in Baltimore, told the Times. "They know someone who had a severe case or died. They are fatigued and want to get back to their normal lives."
Media campaigns, including on-camera moments with politicians and scientists—such as Vice President Mike Pence, President-Elect Joe Biden and Dr. Anthony Fauci—all rolling up their sleeves for the shots may have also helped boost acceptance.
Still, large pockets of skepticism and resistance to vaccination remain. According to the Times, mistrust of the vaccine is higher among Blacks than whites, among Republicans compared to Democrats, and among people living in rural areas versus those in cities.
Still, resistance is fading slowly among most groups, the Times said.
One Black American, Mike Brown, runs a barbershop in Hyattsville, Md. This summer he said he wouldn't get any COVID-19 vaccine, but has since changed his mind.
"The news that it was 95% effective sold me," Brown told the Times. "The side effects sound like what you get after a bad night of drinking and you hurt the next day. Well, I've had many of those and I can deal with that to get rid of the face masks."
More vaccines coming
The U.S. supply of COVID-19 vaccines got a boost last week: Pfizer Inc. and the Trump administration were close to a deal on Tuesday that would get more of the company's coronavirus vaccine to Americans in the coming year.
Such an agreement would help the United States manage a coming vaccine shortage that could leave up to 110 million Americans uncovered in the first half of 2021, the Times reported.
So far, only two pharmaceutical companies—Pfizer and Moderna—have won emergency approval for their COVID-19 vaccines. In the Pfizer negotiations, the government is asking for 100 million additional doses from April through June. The company has indicated it could produce at least 70 million more doses if it can get more supplies and raw materials, the Times reported.
The deal calls for the government to invoke the Defense Production Act to give the company better access to roughly nine specialized products it needs to make the vaccine. One person familiar with the list said it included the lipids that encase the RNA material in both the Moderna and Pfizer vaccines, the Times reported.
Moderna and other companies that have worked more closely with Operation Warp Speed to develop their vaccines already receive favored treatment from suppliers, the Times reported. That includes two companies—Sanofi and Novavax—that have yet to begin large clinical trials in the United States.
Pfizer has already contracted to deliver 100 million doses of its vaccine by the end of March. Moderna has the same agreement, and it has also pledged to sell the government 100 million more doses in the second quarter of the year, the Times reported.
Because the Pfizer and the Moderna vaccines both require two doses, that supply would cover only 150 million Americans out of the roughly 260 million who are eligible to be vaccinated, the newspaper said.
If Pfizer provides another 100 million doses, that would leave only about 60 million eligible Americans uncovered in the first half of the year, the Times reported. Other producers could also step in and cover the shortfall should their vaccines prove successful.
Experts say new COVID variant may already be in U.S.
But as the Pfizer and Moderna vaccines are shipped across the country, U.S. experts warned that the new, more infectious variant of the coronavirus recently discovered in Britain may already be circulating in the United States.
"We don't know that for absolutely certain, but it is reasonable to assume that is going on," Dr. Anthony Fauci, director of the U.S. National Institute of Allergy and Infectious Diseases, said last week. "It very well might be here for all we know."
Several infectious disease experts said last week that the variant may not have even originated in the United Kingdom, the Washington Post reported. Instead, it may have been identified there first because the British government has a strong monitoring system that has tracked tens of thousands of genomic sequences of virus samples. The United States has lagged in sequencing and does not have nearly the same level of virus surveillance, the newspaper said.
"It may very well be here. It may have even started here. The sequencing in the U.S. is so sporadic," Jeremy Luban, a virologist at the University of Massachusetts Medical School, told the Post.
According to Angela Rasmussen, a virologist at the Georgetown Center for Global Health Science and Security, in Washington, D.C., "It makes sense that it was detected first in the U.K. because they have probably the world's best surveillance program. It would not shock me at all to find out that it also is circulating in the U.S."
Even though this variant, officially known as B.1.1.7, is concerning and will require close monitoring, it is unlikely to undermine the United States' mass coronavirus vaccination campaign, stressed William Hanage, an epidemiologist at the Harvard T.H. Chan School of Public Health in Boston.
"The vaccine is a pretty thorough thing," Hanage explained. "Whether or not the existing vaccines are less effective against B.1.1.7 is at the moment not known. I think there is good reason to think they will not be severely impacted."
On Thursday, the U.S. Centers for Disease Control and Prevention announced a new rule that air passengers coming to the United States from the United Kingdom must provide documentation proving that they have tested negative for the new coronavirus within 72 hours of departure.
A global scourge
By Monday, the U.S. coronavirus case count passed 19.1 million while the death toll passed 333,000, according to a Times tally. On Monday, the top five states for coronavirus infections were: California with nearly 2.2 million cases; Texas with close to 1.7 million cases; Florida with almost 1.3 million cases; Illinois with over 939,000 cases; and New York with more than 928,000 cases.
Curbing the spread of the coronavirus in the rest of the world remains challenging.
In India, the coronavirus case count was over 10.2 million on Monday, a Johns Hopkins University tally showed. Brazil had over 7.4 million cases and over 191,000 deaths as of Monday, the Hopkins tally showed.
Worldwide, the number of reported infections passed 80.8 million on Monday, with nearly 1.8 million deaths recorded, according to the Hopkins tally.
More information: The U.S. Centers for Disease Control and Prevention has more on the new coronavirus.
Copyright © 2020 HealthDay. All rights reserved.