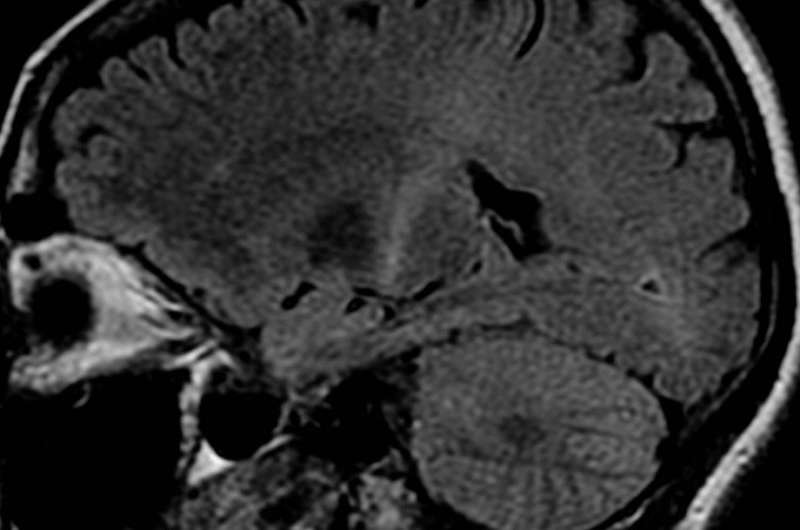
An MRI with increased signal in the posterior part of the internal capsule which can be tracked to the motor cortex consistent with the diagnosis of ALS. Credit: Frank Gaillard/Wikipedia
The antiepileptic drug ezogabine reduced pathologic excitability of cortical and spinal motor neuron cells that are early signs of clinical dysfunction in people with amyotrophic lateral sclerosis (ALS), according to a study conducted by the Neurological Clinical Research Institute of Massachusetts General Hospital (MGH). In addition to providing a clearer understanding of motor neuron excitability as an important disease pathway for ALS, the multi-site study, published in JAMA Neurology, involves the first clinical investigation of ALS (also known as Lou Gehrig's disease) using a drug identified through an induced pluripotent stem cell (iPSC) model.
"The stem cell approach allowed us to capture the hyperexcitability of motor neurons—a prominent disease phenotype—and to then show ezogabine was able to reduce it in people with ALS," says lead author Brian Wainger, MD, Ph.D.>, of the Healey Center for ALS at MGH. "Our findings could have important implications for the field of ALS research both by demonstrating the effect of ezogabine on excitability in people with the disease and by showing that the metrics of cortical and spinal motor neuron excitability may be used as drug biomarkers in multi-site clinical trials."
ALS is a progressive neurodegenerative disorder that leads to the death of neurons in the brain and spinal cord that control speech, swallowing and limb movements. Named after the famous baseball player Lou Gehrig, who was diagnosed with the disease in 1939, there are around 20,000 people in the U.S. with ALS, and another 5,000 newly diagnosed cases each year. Currently, there are three approved drugs in the U.S. for treating ALS, each with limited benefit, creating an urgent need for new therapies that could change the course of the fatal disease.
The MGH study of ezogabine was not designed to assess the long-term effects of the drug on the neurodegenerative disorder, but rather to unravel the biological processes that go awry and identify novel molecular targets for drug intervention. To that end, the ten-week, phase 2 study of 65 participants with ALS at 12 U.S. sites investigated the feasibility of using neuron excitability metrics as predictors of disease progression. "We demonstrated for the first time that these neurophysiological assays can be effectively deployed across multiple study sites, which is important in trials of diseases like ALS where investigators rely on many sites for recruitment," explains Wainger. "That finding could be useful in evaluating other drugs to treat ALS, or even for other diseases where motor neuron metrics could serve as key biomarkers."
Ezogabine (also known as retigabine) had been previously approved by the U.S. Food and Drug Administration (FDA) for treating epilepsy with a unique mechanism of action: facilitating potassium channels in cell membranes that play a central role in controlling neuron excitability, particularly important in the control of seizures. Researchers from MGH's Neurological Clinical Research Institute began evaluating the drug's potential in the context of ALS, using transcranial magnetic stimulation (TMS) and threshold tracking nerve conduction studies (TTNCS) to measure the effects of ezogabine on motor neuron excitability. They learned that ezogabine did indeed calm the excitability of motor neurons.
"Further studies are needed to determine if longer treatment will sustain the effects of reduced excitability and, if so, whether that may slow disease progression," says Wainger. "Through our study we've hopefully established a new research paradigm for using iPSC-based in vitro models for identifying novel disease targets and compounds, and rapidly repurposing drugs for clinical trials."
More information: Brian J. Wainger et al, Effect of Ezogabine on Cortical and Spinal Motor Neuron Excitability in Amyotrophic Lateral Sclerosis, JAMA Neurology (2020). DOI: 10.1001/jamaneurol.2020.4300
Journal information: Archives of Neurology
Provided by Massachusetts General Hospital