Credit: Unsplash/CC0 Public Domain
A pattern in how the brain breaks down tryptophan, a common amino acid consumed through food, was discovered by researchers at The University of Texas Health Science Center at Houston (UTHealth). The finding, which could help physicians more accurately diagnose and treat several major mental health disorders, was recently published in Molecular Psychiatry.
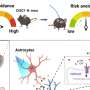
"Tryptophan can be metabolized to either a route where serotonin is produced, or to the kynurenine pathway," said Brisa Fernandes, MD, MSc, Ph.D., a postdoctoral research fellow with the Louis A. Faillace, MD, Department of Psychiatry and Behavioral Sciences at McGovern Medical School at UTHealth and co-senior author of the paper. "The research shows that people with mood disorders and schizophrenia not only have decreased levels of tryptophan overall, but the tryptophan they do have is being broken down more often in the kynurenine pathway, shifting away from the production of serotonin, the chemical made from tryptophan that is thought to regulate anxiety and improve mood."
Researchers conducted a systematic review of more than 100 peer-reviewed studies assessing kynurenine metabolites, which are the tryptophan amino acids being broken down in the kynurenine pathway, in people with major depressive disorder, schizophrenia, or bipolar disorder, compared to healthy controls. Within those studies, which included more than 10,000 study participants, they examined the difference in metabolite concentrations between each group.
"We chose these disorders because they share several biological pathways, meaning the same root causes and pathophysiology. We tried to find biomarkers that are common among these disorders, but most importantly, biomarkers that distinguish them. This is essential to help clinicians guide treatment and implement personalized medicine in psychiatry—not the 'one-size-fits-all' approach that is so prevalent today," Fernandes said.
As the tryptophan breaks down in the brain, it can use the kynurenine pathway to turn into quinolinic acid, which is considered neurotoxic, or kynurenic acid, which is considered neuroprotective.
Researchers identified several patterns that occur as tryptophan breaks down that collectively suggest there is a shift from serotonin production to the kynurenine pathway, which could lead to increased neurotoxicity from the quinolinic acid.
"This can help us to develop new tests for diagnosing these disorders and, more importantly, of discovering and selecting new treatments for people with mood disorders and schizophrenia that will be more personalized," Fernandes said.
"The major challenge that we have in psychiatry is having biomarkers to differentiate one diagnostic from the other, as our diagnoses are 100% clinical. This is the type of research we need to identify those biomarkers, and the next step for us now is to see if we can validate these findings in our patients," said João de Quevedo, MD, Ph.D., professor of psychiatry and behavioral sciences and co-author of the study.
"This is important research that hopefully will lead to a better understanding of mechanisms involved in major mental illnesses, as well as a higher level of personalization in treatments so we can best match patients to a treatment plan we know will help them," said Jair Soares, MD, Ph.D., the Pat R. Rutherford, Jr. Chair in Psychiatry in the Faillace Department of Psychiatry and Behavioral Sciences and co-senior author. Soares and de Quevedo see patients at UT Physicians, the clinical practice of McGovern Medical School.
More information: Wolfgang Marx et al, The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: a meta-analysis of 101 studies, Molecular Psychiatry (2020). DOI: 10.1038/s41380-020-00951-9
Journal information: Molecular Psychiatry