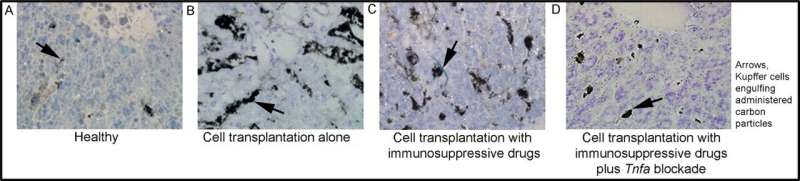
Activation of monocytes (Kupffer cells) was determined with a pulse of carbon (arrows, black grains). A: Healthy liver with limited carbon incorporation indicates most Kupffer cells are inactive. B: Liver showing an example of extensive carbon uptake six hours after cell transplantation. C: Cell transplantation in the presence of immunosuppression with mycophenolate mofetil and tacrolimus reveals that Kupffer cells remain active. D. Cell transplantation with immunosuppressive drugs plus blocking of TNF prevents Kupffer cell activation. Original magnification, x200. Toluidine blue counterstain. Credit: Albert Einstein College of Medicine
Controlling inflammation after transplantation of organs, cells, or tissues is critical for graft survival; however, it can be difficult. Continuing injuries due to chronic rejection can be particularly problematic. Now, a team of researchers from Albert Einstein College of Medicine report that neutralizing the cell signaling molecule, tumor necrosis factor (TNF), can prevent cascades of injurious molecules and signals after cell transplantation in The American Journal of Pathology.
"New insights into immune inflammatory mediators indicate that rejection and tissue injury can be avoided with available drugs," explained director of the research team Sanjeev Gupta, MD, Department of Medicine and of Pathology, and Marion Bessin Liver Research Center, Albert Einstein College of Medicine, Bronx, NY, USA. "The ability to prevent and/or control tissue injury will improve graft survival for transplantation medicine, advance use of cells and tissues for regenerative medicine, and offer insights for controlling inflammation-related tissue injury in other circumstances."
Transplanting organs from unrelated or mismatched donors into individuals triggers immunological defenses against 'foreign' material. Development of immunosuppressive drugs to block lymphocyte responses has delivered success in the short term, but chronic rejection and tissue injury over long periods due to unidentified mechanisms and processes can lead to the loss of transplanted organs.
To understand the factors at work in tissue rejection, the researchers at Albert Einstein College of Medicine developed liver cell transplantation models in laboratory rats with or without immunosuppression, using drugs routinely given to humans. The state of inflammation was studied using gene expression arrays to query the activation of soluble signals associated with various cell types. The analysis was guided by recent advances in mapping gene expression differences and the networks affected during complex interactions using commercially available computer software. The software queries the scientific literature to suggest relationships and negative or positive reactions in genes.
The studies identified differences in the expression of 40-50 soluble networks. In particular, the activation of TNF, a major inflammatory mediator, and its cellular receptors is prominent, persisting over the long term.
Blocking TNF stopped the activation of most other inflammatory signals, revealing that it is a "master switch" for orchestrating cytokines, chemokines, and receptors arising from the body's innate or inbuilt systems defending against foreign material, e.g., microbes, viruses, and mismatched transplants. To further understand the role of TNF, the researchers neutralized it with the well-established drugs etanercept or thalidomide to improve cell transplant outcomes alongside conventional immunosuppression with tacrolimus and mycophenolate mofetil.
Computer mapping of gene expression showed that blocking TNF markedly decreases the recruitment and activity of inflammatory cells. Other studies provided direct evidence that blocking TNF led to superior survival and proliferation of transplanted cells, regenerating the liver far more effectively.
Lead author Fadi-Luc Jaber, Ph.D., Marion Bessin Liver Research Center, Albert Einstein College of Medicine, Bronx, NY, USA, commented "The efficacy of this anti-inflammatory mechanism for liver regeneration surprised me. These mechanisms will provide further means to treat liver conditions."
"The translational potential of these findings is exciting because the additional inflammatory markers and genes offer further means for diagnosing the pathology of organ rejection in individuals. This type of inflammatory interference will decrease tissue injury, promote graft survival, and survival of transplanted cells and engineered tissues, and help advance organ regeneration," observed Dr. Gupta. "The inflammatory cascades orchestrated through TNF also hold lessons for the pathology in other less well understood conditions, such as COVID-19."
More information: Fadi Luc Jaber et al, Tumor Necrosis Factor Directs Allograft-Related Innate Responses and Its Neutralization Improves Hepatocyte Engraftment in Rats, The American Journal of Pathology (2020). DOI: 10.1016/j.ajpath.2020.09.014
Journal information: American Journal of Pathology
Provided by Elsevier