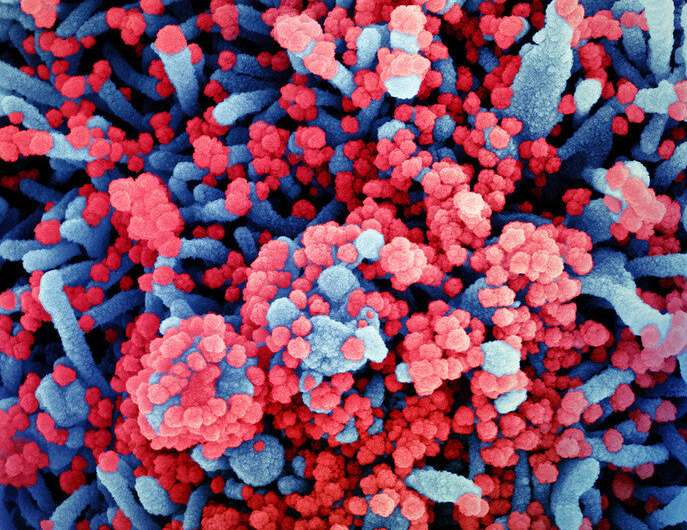
Colorized scanning electron micrograph of a cell (blue) heavily infected with SARS-CoV-2 virus particles (red), isolated from a patient sample. Image captured at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Credit: NIAID
A lot has been written recently about the merits of different potential vaccines for SARS-CoV-2. "Merit" is generally assumed to mean that the vaccine will make us immune. But what, exactly, is immunity, and how would we attempt to measure it once attained? The short answer is that we don't really measure immunity. The longer answer is that sometimes we do at least have some clues.
One such clue is the actual antibody response to an infection, or by implication, the antibody response to a potential vaccine against it. But which responding antibodies, and to which parts of the virus or bacteria are we talking about? Etymologically speaking, immune simply means exempt—an exemption from having to mount a response. This reaction, in turn, being a response by fire, or rather, inflammation.
Two papers recently published in Science Translational Medicine characterize the antibody response to infection with the SARS virus. Normally, this initially consists of a primary IgM antibody response, which is then followed by a secondary IgG, IgA and IgE response that contributes to immune memory. In the first study, however, Sterlin et al. measured the acute (short term) humoral response to SARS infection and found that it was instead dominated by IgA antibodies. They measured both the increase in the number of antibody-producing cells, and also the total neutralizing antibody levels found in serum, saliva and broncho-alveolar fluid of patients. In theory, neutralizing antibodies are those which can actually neutralize the virus instead of just sticking to it, therefore blocking the disease.
In practice, the full nasty is rarely used in the lab. Instead of full-blown bio-infectious SARS, researchers in BSl-2 labs (as opposed to BSL-4 labs) use something called a pseudovirus. This is essentially a SARS virus that has been crippled somehow so that it is no longer a real threat. This can be done by encapsulating the genome of the virus within the protein coat of some other kind of virus. Scientists can also modify or inactivate surface proteins by other means, or provide necessary components separately by using some kind of helper virus. Typically, once inside a susceptible cell, a pseudovirus will at best be able to replicate a single time.
The researchers found that while the other Ig components were eventually detected, it was the IgA antibodies that shouldered the load. IgA serum concentrations decreased notably by one month post-infection, but neutralizing IgA remained detectable in saliva for up to 73 days. They note that these results raise critical questions about which types of antibodies that vaccine regimens should be targeting to prevent either infection or re-infection.
In the second paper, authors Wang et. al. come to similar conclusions. They also further demonstrate that dimerization of IgA increases its potency over the monomeric form. The mucosal form of IgA appears predominantly as a dimer of two IgA monomers covalently linked together by J chain. While they do not explain how all this happens, they note that subsequent crosslinking of the spike protein on the viral surface enhances neutralizing ability either directly, or by some other method of increasing apparent affinity.
Similarly, it seems that monovalent Fab fragments of serum IgG antibodies are far less potent then the full antibody. The authors additionally propose that the increased flexibility and longer hinge of IgA1 subtype, relative to the IgG, would have better interaction with SARS spike tri-mers.
Looking past the critical receptor binding domain of the spike protein, the RBD, many other possible antibody targets are now coming into better view. Recent study of the human leukocyte antigen Class II immunopeptidome has revealed that dendritic cells of the immune system display peptide fragments that span the entire spike protein. The authors also found that while HLA-II peptides from 11 locations where presented in most of the analyzed donors, their correlation to the predicted peptide fragments was low.
Proteomics studies, like the immunopeptidome work, have been enjoying the spotlight in recent times. If the number of pictures of so-called volcano plots depicting changes in protein levels featured in Phys.org articles is any indicator of the times, then Monday in particular was a very explosive day. However, a curious paper was recently published about the formation of protein dimers, much like the IgA under discussion here. It made the claim that a simple rule drives the evolution of what they call useless complexity. In other words, protein complexes, like dimers, don't arise as an increase in fitness, but rather come about from an inexorable process they have dubbed the hydrophobic ratchet.
Their ratchet demands that when mutations inevitably occur and create new hydrophobic regions in proteins, they respond by clumping together into complexes without necessarily increasing any useful function. Since otherwise deleterious mutations can effectively be hidden at these interfaces, it is now correspondingly harder to get rid of these complexes since they are not exposed to purifying selection forces—if, that is, you believe that this mysterious purifying force really exists. As Luca Turin perhaps poignantly observed on Twitter yesterday: "There goes half of proteomics."
For now, the question of IgA dimerization may await further study. As peer-reviewed results of new vaccines, like the mRNA vaccines from Pfizer and Moderna, or the viral-vector-based vaccines from Oxford/Astrazeneca begin to trickle out into the blogosphere and beyond, important questions remain.
Pundits sometimes still seem to be arguing over whether the initial rollout of the vaccine should be a privilege or a punishment. For example, while many argue for special advanced access to the underserved, just as many see this as problematic. Over at the bastion of common sense at Cornell's Health Department, they have already gone so far as to say that anyone identifying as a person of color who perceives historical injustices can covertly request to be exempt from any of their new mandatory vaccine requirements. In the greater scheme of things, vaccine immunity could turn out to be an invaluable thing to have. Elsewhere, a few others have suggested that everyone should probably just be treated as equals, at least when it comes to experimental new vaccines.
More information: Delphine Sterlin et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2, Science Translational Medicine (2020). DOI: 10.1126/scitranslmed.abd2223
Zijun Wang et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA, Science Translational Medicine (2020). DOI: 10.1126/scitranslmed.abf1555
Journal information: Science Translational Medicine
© 2020 Science X Network